J Korean Neurosurg Soc.
2014 Mar;55(3):131-135. 10.3340/jkns.2014.55.3.131.
Targeting Orthotopic Glioma in Mice with Genetically Engineered Salmonella typhimurium
- Affiliations
-
- 1Brain Tumor Research Laboratory and Department of Neurosurgery, Chonnam National University Research Institute, Chonnam National University Hwasun Hospital and Medical School, Hwasun, Korea. sjung@chonnam.ac.kr
- 2Laboratory of In Vivo Molecular Imaging (LOVMI) and Department of Nuclear Medicine, Chonnam National University Research Institute, Chonnam National University Hwasun Hospital and Medical School, Hwasun, Korea.
- KMID: 2191077
- DOI: http://doi.org/10.3340/jkns.2014.55.3.131
Abstract
OBJECTIVE
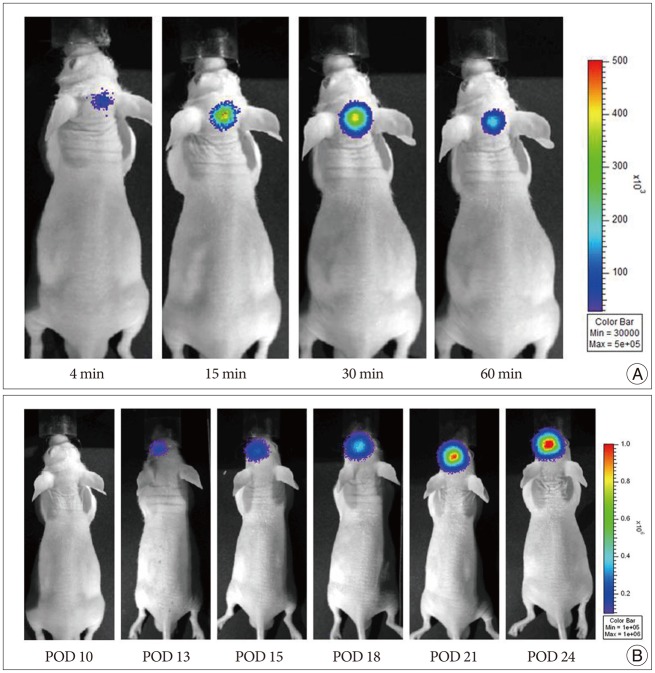
With the growing interests of bacteria as a targeting vector for cancer treatment, diverse genetically engineered Salmonella has been reported to be capable of targeting primary or metastatic tumor regions after intravenous injection into mouse tumor models. The purpose of this study was to investigate the capability of the genetically engineered Salmonella typhimurium (S. typhimurium) to access the glioma xenograft, which was monitored in mouse brain tumor models using optical bioluminescence imaging technique.
METHODS
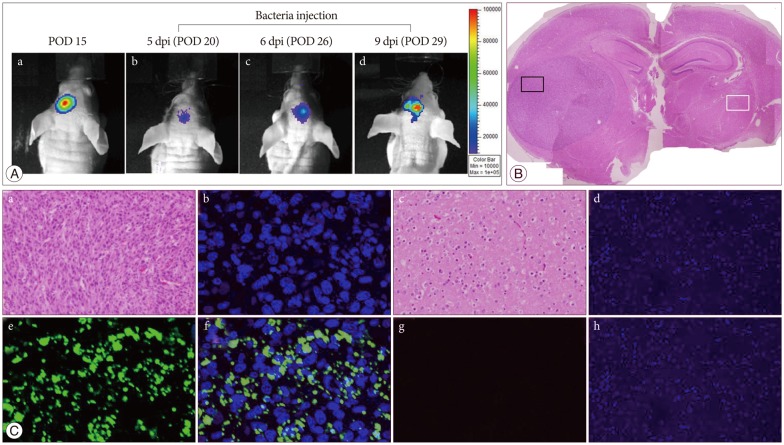
U87 malignant glioma cells (U87-MG) stably transfected with firefly luciferase (Fluc) were implanted into BALB/cAnN nude mice by stereotactic injection into the striatum. After tumor formation, attenuated S. typhimurium expressing bacterial luciferase (Lux) was injected into the tail vein. Bioluminescence signals from transfected cells or bacteria were monitored using a cooled charge-coupled device camera to identify the tumor location or to trace the bacterial migration. Immunofluorescence staining was also performed in frozen sections of mouse glioma xenograft.
RESULTS
The injected S. typhimurium exclusively localized in the glioma xenograft region of U87-MG-bearing mouse. Immunofluorescence staining also demonstrated the accumulation of S. typhimurium in the brain tumors.
CONCLUSION
The present study demonstrated that S. typhimurium can target glioma xenograft, and may provide a potentially therapeutic probe for glioma.
Keyword
MeSH Terms
Figure
Reference
-
1. Dinca EB, Voicu RV, Ciurea AV. Bioluminescence imaging of invasive intracranial xenografts : implications for translational research and targeted therapeutics of brain tumors. Neurosurg Rev. 2010; 33:385–394. PMID: 20652720.
Article2. Hoffman S, Propp JM, McCarthy BJ. Temporal trends in incidence of primary brain tumors in the United States, 1985-1999. Neuro Oncol. 2006; 8:27–37. PMID: 16443945.
Article3. Min JJ, Kim HJ, Park JH, Moon S, Jeong JH, Hong YJ, et al. Noninvasive real-time imaging of tumors and metastases using tumor-targeting light-emitting Escherichia coli. Mol Imaging Biol. 2008; 10:54–61. PMID: 17994265.
Article4. Min JJ, Nguyen VH, Kim HJ, Hong Y, Choy HE. Quantitative bioluminescence imaging of tumor-targeting bacteria in living animals. Nat Protoc. 2008; 3:629–636. PMID: 18388945.
Article5. Momiyama M, Zhao M, Kimura H, Tran B, Chishima T, Bouvet M, et al. Inhibition and eradication of human glioma with tumor-targeting Salmonella typhimurium in an orthotopic nude-mouse model. Cell Cycle. 2012; 11:628–632. PMID: 22274398.
Article6. Nguyen VH, Kim HS, Ha JM, Hong Y, Choy HE, Min JJ. Genetically engineered Salmonella typhimurium as an imageable therapeutic probe for cancer. Cancer Res. 2010; 70:18–23. PMID: 20028866.
Article7. Pawelek JM, Low KB, Bermudes D. Bacteria as tumour-targeting vectors. Lancet Oncol. 2003; 4:548–556. PMID: 12965276.
Article8. Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997; 57:4537–4544. PMID: 9377566.9. Rehemtulla A, Stegman LD, Cardozo SJ, Gupta S, Hall DE, Contag CH, et al. Rapid and quantitative assessment of cancer treatment response using in vivo bioluminescence imaging. Neoplasia. 2000; 2:491–495. PMID: 11228541.
Article10. Szentirmai O, Baker CH, Lin N, Szucs S, Takahashi M, Kiryu S, et al. Noninvasive bioluminescence imaging of luciferase expressing intracranial U87 xenografts : correlation with magnetic resonance imaging determined tumor volume and longitudinal use in assessing tumor growth and antiangiogenic treatment effect. Neurosurgery. 2006; 58:365–372. discussion 365-372. PMID: 16462491.
Article11. Tang Y, Shah K, Messerli SM, Snyder E, Breakefield X, Weissleder R. In vivo tracking of neural progenitor cell migration to glioblastomas. Hum Gene Ther. 2003; 14:1247–1254. PMID: 12952596.12. Yu YA, Shabahang S, Timiryasova TM, Zhang Q, Beltz R, Gentschev I, et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol. 2004; 22:313–320. PMID: 14990953.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Targeted Cancer Therapy Using Engineered Salmonella typhimurium
- Multidrug-resistant Salmonella typhimurium and Salmonella enteritidis Identified by Multiplex PCR in Korea
- Electron microscopic study on the response of the intestinal mucosa and macrophage to invasion of salmonella typhimurium
- In vitro infection of murine macrophages with salmonella typhimurium and listeria monocytogenes
- Investigation of antigen related to the in vitro invasiveness of salmonella typhimurium through the Madin-Darby canine kidney(MDCK) epithelial cell monolayer