J Korean Neurosurg Soc.
2015 May;57(5):323-328. 10.3340/jkns.2015.57.5.323.
Combination Therapy for Gliomas Using Temozolomide and Interferon-Beta Secreting Human Bone Marrow Derived Mesenchymal Stem Cells
- Affiliations
-
- 1Department of Neurosurgery, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea. ssjeun@catholic.ac.kr
- 2Department of Biomedical Science, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 1881728
- DOI: http://doi.org/10.3340/jkns.2015.57.5.323
Abstract
OBJECTIVE
Malignant gliomas are the most common primary tumors of the central nervous system and the prognosis of patients with gliomas is poor. The combination of interferon-bata (IFN-beta) and temozolomide (TMZ) has shown significant additive antitumor effects in human glioma xenograft models. Considering that the poor survival of patients with human malignant gliomas relates partly to the inability to deliver therapeutic agents to the tumor, the tropism of human bone marrow-derived mesenchymal stem cells (MSC) for malignant gliomas can be exploited to therapeutic advantages. We investigated the combination effects of TMZ and MSCs that secrete IFN-beta on gliomas.
METHODS
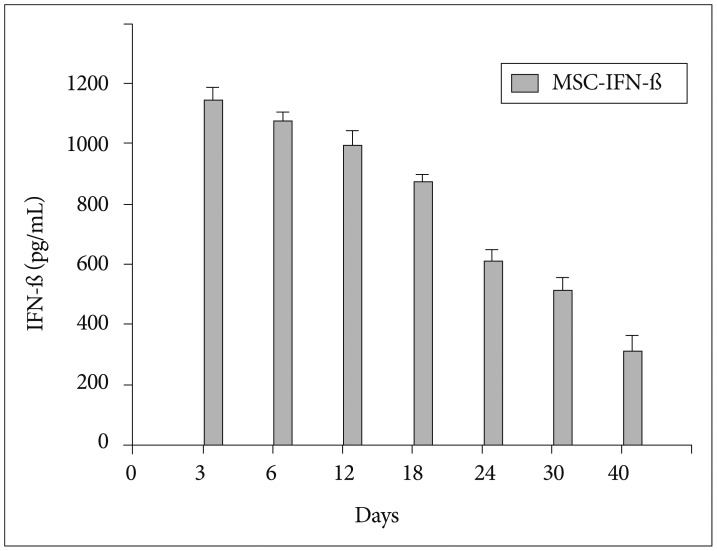
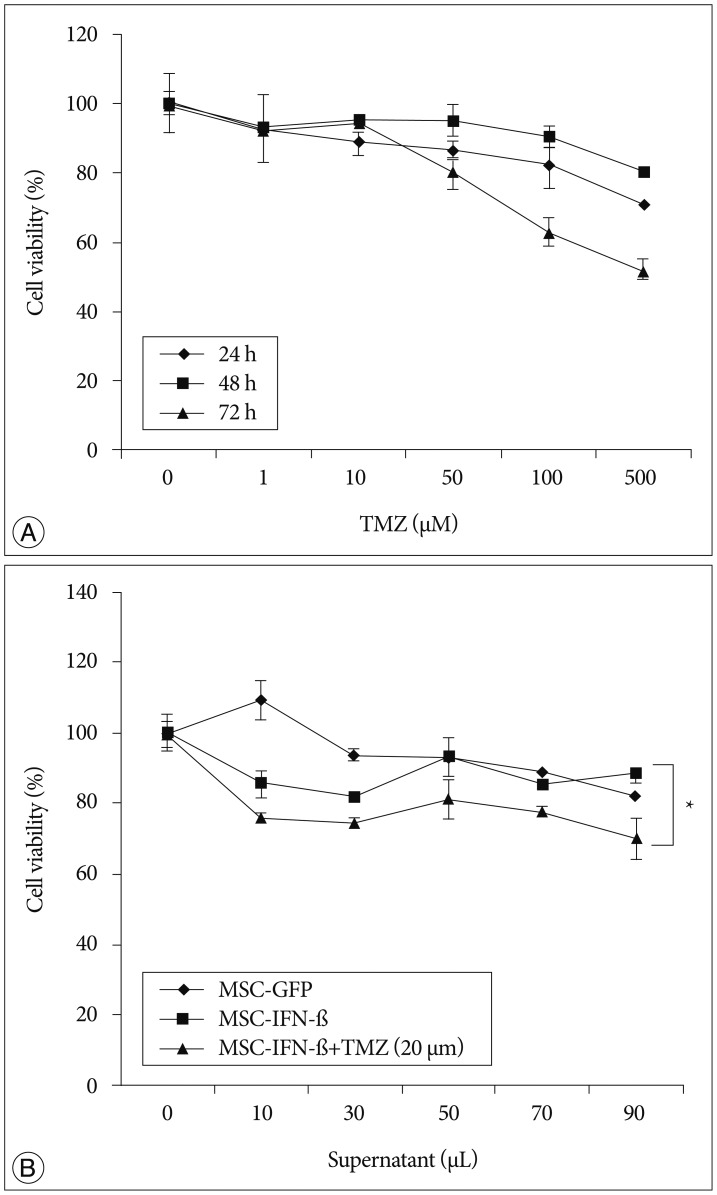
We engineered human MSCs to secret mouse IFN-beta (MSC-IFN-beta) via adenoviral transduction and confirmed their secretory capacity using enzyme-linked immunosorbent assays. In vitro and in vivo experiments were performed to determine the effects of the combined TMZ and MSC-IFN-beta treatment.
RESULTS
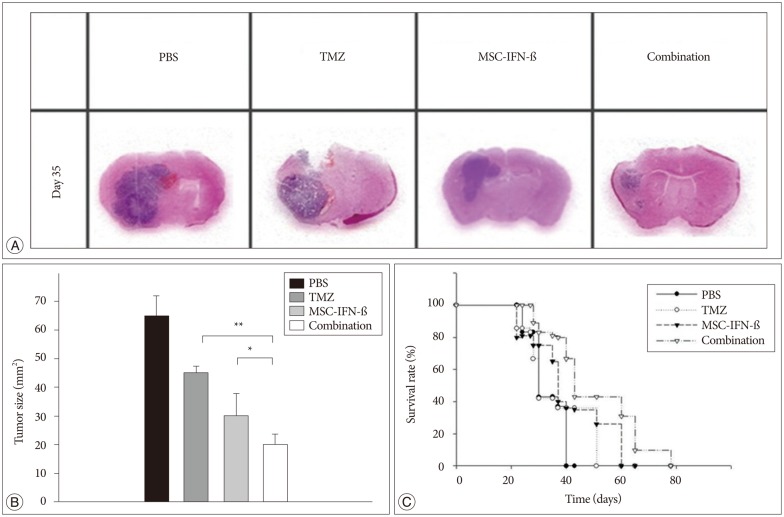
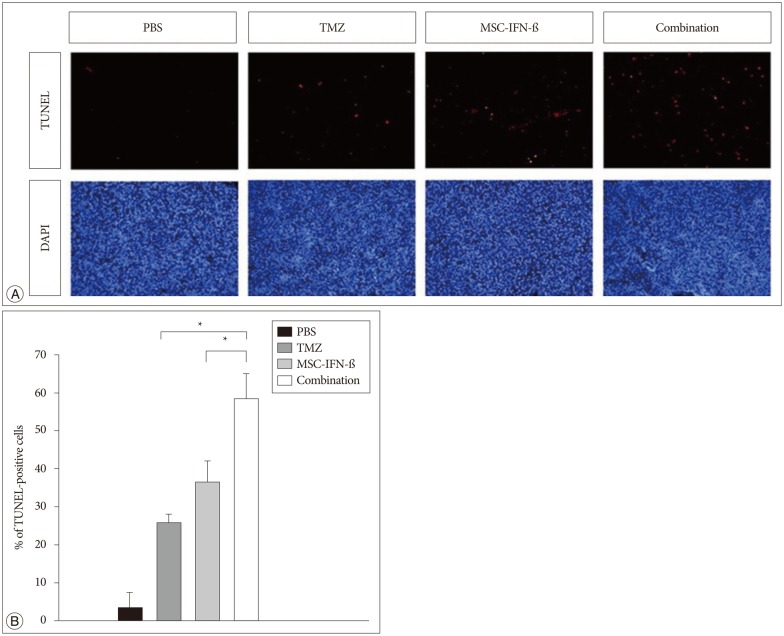
In vitro, the combination of MSC-IFN-beta and TMZ showed significantly enhanced antitumor effects in GL26 mouse glioma cells. In vivo, the combined MSC-IFN-beta and TMZ therapy significantly reduced the tumor size and improved the survival rates compared to each treatment alone.
CONCLUSION
These results suggest that MSCs can be used as an effective delivery vehicle so that the combination of MSC-IFN-beta and TMZ could be considered as a new option for the treatment of malignant gliomas.
Keyword
MeSH Terms
Figure
Reference
-
1. Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay : assessment of chemosensitivity testing. Cancer Res. 1987; 47:936–942. PMID: 3802100.2. Ho IA, Toh HC, Ng WH, Teo YL, Guo CM, Hui KM, et al. Human bone marrow-derived mesenchymal stem cells suppress human glioma growth through inhibition of angiogenesis. Stem Cells. 2013; 31:146–155. PMID: 23034897.
Article3. Jin HT, Youn JI, Kim HJ, Lee JB, Ha SJ, Koh JS, et al. Enhancement of interleukin-12 gene-based tumor immunotherapy by the reduced secretion of p40 subunit and the combination with farnesyltransferase inhibitor. Hum Gene Ther. 2005; 16:328–338. PMID: 15812228.
Article4. Kim SM, Lim JY, Park SI, Jeong CH, Oh JH, Jeong M, et al. Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Res. 2008; 68:9614–9623. PMID: 19047138.
Article5. Lawrence YR, Mishra MV, Werner-Wasik M, Andrews DW, Showalter TN, Glass J, et al. Improving prognosis of glioblastoma in the 21st century : who has benefited most? Cancer. 2012; 118:4228–4234. PMID: 22180310.
Article6. Nakamizo A, Marini F, Amano T, Khan A, Studeny M, Gumin J, et al. Human bone marrow-derived mesenchymal stem cells in the treatment of gliomas. Cancer Res. 2005; 65:3307–3318. PMID: 15833864.
Article7. Natsume A, Ishii D, Wakabayashi T, Tsuno T, Hatano H, Mizuno M, et al. IFN-beta down-regulates the expression of DNA repair gene MGMT and sensitizes resistant glioma cells to temozolomide. Cancer Res. 2005; 65:7573–7579. PMID: 16140920.
Article8. Natsume A, Wakabayashi T, Ishii D, Maruta H, Fujii M, Shimato S, et al. A combination of IFN-beta and temozolomide in human glioma xenograft models : implication of p53-mediated MGMT downregulation. Cancer Chemother Pharmacol. 2008; 61:653–659. PMID: 17564708.
Article9. Ryu CH, Yoon WS, Park KY, Kim SM, Lim JY, Woo JS, et al. Valproic acid downregulates the expression of MGMT and sensitizes temozolomide-resistant glioma cells. J Biomed Biotechnol. 2012; 2012:987495. PMID: 22701311.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients
- Bone marrow-derived stem cells contribute to regeneration of the endometrium
- Bone Marrow-Derived Mesenchymal Stem Cells for Regenerative Medicine
- Combination Cell Therapy with Mesenchymal Stem Cells and Neural Stem Cells for Brain Stroke in Rats