Int J Stem Cells.
2024 Aug;17(3):270-283. 10.15283/ijsc24060.
Glutathione Dynamics in the Tumor Microenvironment: A Potential Target of Cancer Stem Cells and T Cells
- Affiliations
-
- 1Jeju Research Institute of Pharmaceutical Sciences, College of Pharmacy, Jeju National University, Jeju, Korea
- KMID: 2558605
- DOI: http://doi.org/10.15283/ijsc24060
Abstract
- Glutathione (GSH), the main cellular antioxidant, dynamically influences tumor growth, metastasis, and resistance to therapy in the tumor microenvironment (TME), which comprises cancer cells, immune cells, stromal cells, and non-cellular components, including the extracellular matrix, metabolites, hypoxia, and acidity. Cancer stem cells (CSCs) and T cells are minor but significant cell subsets of the TME. GSH dynamics influences the fate of CSCs and T cells. Here, we explored GSH dynamics in CSCs and T cells within the TME, as well as therapeutic approaches that could target these dynamics.
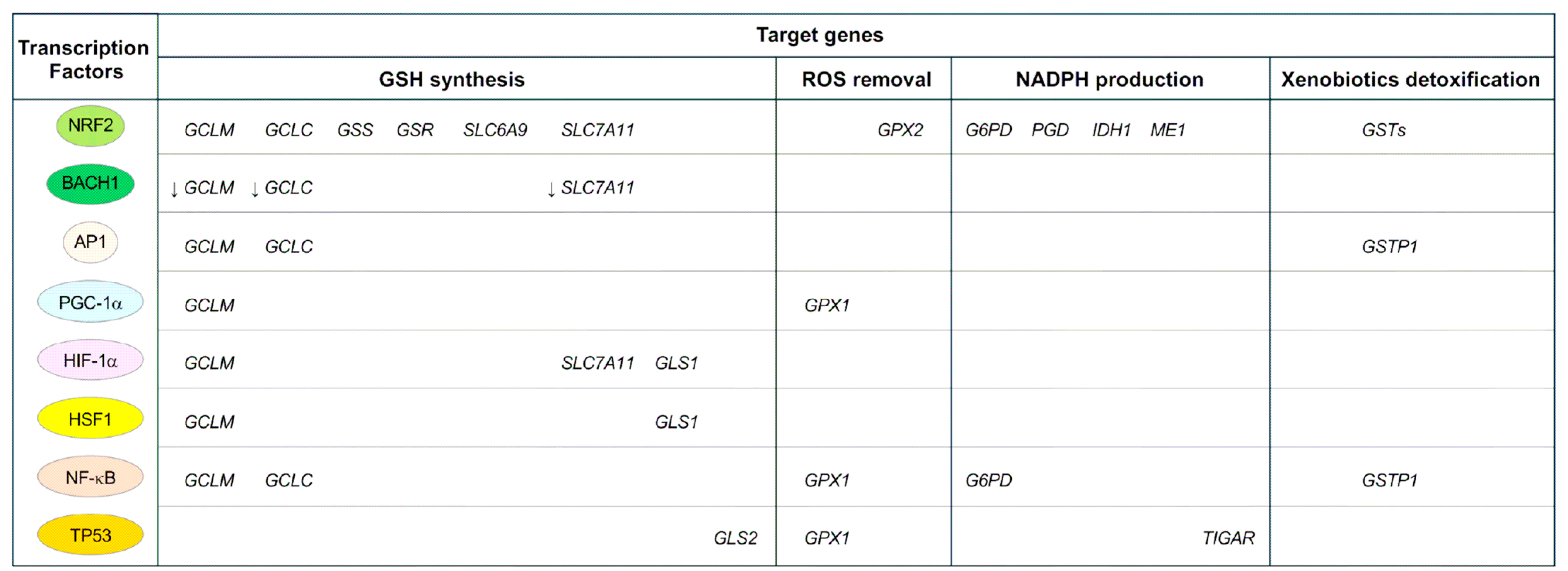
Figure
Reference
-
References
1. Albini A, Bruno A, Gallo C, Pajardi G, Noonan DM, Dallaglio K. 2015; Cancer stem cells and the tumor microenvironment: interplay in tumor heterogeneity. Connect Tissue Res. 56:414–425. DOI: 10.3109/03008207.2015.1066780. PMID: 26291921. PMCID: PMC4673538.2. Demicco M, Liu XZ, Leithner K, Fendt SM. 2024; Metabolic heterogeneity in cancer. Nat Metab. 6:18–38. DOI: 10.1038/s42255-023-00963-z. PMID: 38267631.3. Lapidot T, Sirard C, Vormoor J, et al. 1994; A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 367:645–648. DOI: 10.1038/367645a0. PMID: 7509044.4. Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. 2003; Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 100:3983–3988. DOI: 10.1073/pnas.0530291100. PMID: 12629218. PMCID: PMC153034.5. Chi H, Pepper M, Thomas PG. 2024; Principles and therapeutic applications of adaptive immunity. Cell. 187:2052–2078. DOI: 10.1016/j.cell.2024.03.037. PMID: 38670065. PMCID: PMC11177542.6. Jiang Y, Li Y, Zhu B. 2015; T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 6:e1792. DOI: 10.1038/cddis.2015.162. PMID: 26086965. PMCID: PMC4669840.7. Tie Y, Tang F, Wei YQ, Wei XW. 2022; Immunosuppressive cells in cancer: mechanisms and potential therapeutic targets. J Hematol Oncol. 15:61. DOI: 10.1186/s13045-022-01282-8. PMID: 35585567. PMCID: PMC9118588.8. Li C, Jiang P, Wei S, Xu X, Wang J. 2020; Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer. 19:116. DOI: 10.1186/s12943-020-01234-1. PMID: 32680511. PMCID: PMC7367382.9. Asadzadeh Z, Mohammadi H, Safarzadeh E, et al. 2017; The paradox of Th17 cell functions in tumor immunity. Cell Immunol. 322:15–25. DOI: 10.1016/j.cellimm.2017.10.015. PMID: 29103586.10. Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, Paulos CM. 2014; Th17 cells in cancer: the ultimate identity crisis. Front Immunol. 5:276. DOI: 10.3389/fimmu.2014.00276. PMID: 24987392. PMCID: PMC4060300.11. Lu SC. 2013; Glutathione synthesis. Biochim Biophys Acta. 1830:3143–3153. DOI: 10.1016/j.bbagen.2012.09.008. PMID: 22995213. PMCID: PMC3549305.12. Jeong EM, Shin JW, Lim J, et al. 2019; Monitoring glutathione dynamics and heterogeneity in living stem cells. Int J Stem Cells. 12:367–379. DOI: 10.15283/ijsc18151. PMID: 30836726. PMCID: PMC6657947.13. Kim J, Gong YX, Jeong EM. 2023; Measuring glutathione regeneration capacity in stem cells. Int J Stem Cells. 16:356–362. DOI: 10.15283/ijsc23047. PMID: 37385637. PMCID: PMC10465335.14. Lim J, Heo J, Ju H, et al. 2020; Glutathione dynamics determine the therapeutic efficacy of mesenchymal stem cells for graft-versus-host disease via CREB1-NRF2 pathway. Sci Adv. 6:eaba1334. DOI: 10.1126/sciadv.aba1334. PMID: 32490200. PMCID: PMC7239701.15. Jeong EM, Yoon JH, Lim J, et al. 2018; Real-time monitoring of glutathione in living cells reveals that high glutathione levels are required to maintain stem cell function. Stem Cell Reports. 10:600–614. DOI: 10.1016/j.stemcr.2017.12.007. PMID: 29307581. PMCID: PMC5830891.16. Zhu Y, Carvey PM, Ling Z. 2006; Age-related changes in glutathione and glutathione-related enzymes in rat brain. Brain Res. 1090:35–44. DOI: 10.1016/j.brainres.2006.03.063. PMID: 16647047. PMCID: PMC1868496.17. Li H, Ning S, Ghandi M, et al. 2019; The landscape of cancer cell line metabolism. Nat Med. 25:850–860. DOI: 10.1038/s41591-019-0404-8. PMID: 31068703. PMCID: PMC6629041.18. Jagust P, Alcalá S, Sainz B Jr, Heeschen C, Sancho P. 2020; Glutathione metabolism is essential for self-renewal and chemoresistance of pancreatic cancer stem cells. World J Stem Cells. 12:1410–1428. DOI: 10.4252/wjsc.v12.i11.1410. PMID: 33312407. PMCID: PMC7705467.19. Mukha A, Kahya U, Linge A, et al. 2021; GLS-driven glutamine catabolism contributes to prostate cancer radiosensitivity by regulating the redox state, stemness and ATG5-mediated autophagy. Theranostics. 11:7844–7868. DOI: 10.7150/thno.58655. PMID: 34335968. PMCID: PMC8315064.20. Polewski MD, Reveron-Thornton RF, Cherryholmes GA, Marinov GK, Aboody KS. 2017; SLC7A11 overexpression in glioblastoma is associated with increased cancer stem cell-like properties. Stem Cells Dev. 26:1236–1246. DOI: 10.1089/scd.2017.0123. PMID: 28610554. PMCID: PMC5576215.21. Xu X, Wang L, Zang Q, et al. 2021; Rewiring of purine metabolism in response to acidosis stress in glioma stem cells. Cell Death Dis. 12:277. DOI: 10.1038/s41419-021-03543-9. PMID: 33723244. PMCID: PMC7961141.22. Asai R, Tsuchiya H, Amisaki M, et al. 2019; CD44 standard isoform is involved in maintenance of cancer stem cells of a hepatocellular carcinoma cell line. Cancer Med. 8:773–782. DOI: 10.1002/cam4.1968. PMID: 30636370. PMCID: PMC6382709.23. Wang SQ, Chen JJ, Jiang Y, et al. 2023; Targeting GSTP1 as therapeutic strategy against lung adenocarcinoma stemness and resistance to tyrosine kinase inhibitors. Adv Sci (Weinh). 10:e2205262. DOI: 10.1002/advs.202205262. PMID: 36709476. PMCID: PMC9982593.24. Amaya ML, Inguva A, Pei S, et al. 2022; The STAT3-MYC axis promotes survival of leukemia stem cells by regulating SLC1A5 and oxidative phosphorylation. Blood. 139:584–596. DOI: 10.1182/blood.2021013201. PMID: 34525179. PMCID: PMC8796651.25. Hughes CE, Coody TK, Jeong MY, Berg JA, Winge DR, Hughes AL. 2020; Cysteine toxicity drives age-related mitochondrial decline by altering iron homeostasis. Cell. 180:296–310.e18. DOI: 10.1016/j.cell.2019.12.035. PMID: 31978346. PMCID: PMC7164368.26. Ju HQ, Lu YX, Chen DL, et al. 2016; Redox regulation of stem-like cells though the CD44v-xCT axis in colorectal cancer: mechanisms and therapeutic implications. Theran-ostics. 6:1160–1175. DOI: 10.7150/thno.14848. PMID: 27279909. PMCID: PMC4893643.27. Ishimoto T, Nagano O, Yae T, et al. 2011; CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(-) and thereby promotes tumor growth. Cancer Cell. 19:387–400. DOI: 10.1016/j.ccr.2011.01.038. PMID: 21397861.28. Ogihara K, Kikuchi E, Okazaki S, et al. 2019; Sulfasalazine could modulate the CD44v9-xCT system and enhance cisplatin-induced cytotoxic effects in metastatic bladder cancer. Cancer Sci. 110:1431–1441. DOI: 10.1111/cas.13960. PMID: 30719824. PMCID: PMC6447829.29. Bensaad K, Tsuruta A, Selak MA, et al. 2006; TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 126:107–120. DOI: 10.1016/j.cell.2006.05.036. PMID: 16839880.30. Cheung EC, Ludwig RL, Vousden KH. 2012; Mitochondrial localization of TIGAR under hypoxia stimulates HK2 and lowers ROS and cell death. Proc Natl Acad Sci U S A. 109:20491–20496. DOI: 10.1073/pnas.1206530109. PMID: 23185017. PMCID: PMC3528527.31. Maddocks OD, Berkers CR, Mason SM, et al. 2013; Serine starvation induces stress and p53-dependent metabolic remodelling in cancer cells. Nature. 493:542–546. DOI: 10.1038/nature11743. PMID: 23242140. PMCID: PMC6485472.32. Hayes JD, Dinkova-Kostova AT, Tew KD. 2020; Oxidative stress in cancer. Cancer Cell. 38:167–197. DOI: 10.1016/j.ccell.2020.06.001. PMID: 32649885. PMCID: PMC7439808.33. Piskounova E, Agathocleous M, Murphy MM, et al. 2015; Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 527:186–191. DOI: 10.1038/nature15726. PMID: 26466563. PMCID: PMC4644103.34. He C, Danes JM, Hart PC, et al. 2019; SOD2 acetylation on lysine 68 promotes stem cell reprogramming in breast cancer. Proc Natl Acad Sci U S A. 116:23534–23541. DOI: 10.1073/pnas.1902308116. PMID: 31591207. PMCID: PMC6876149.35. Yoo HC, Park SJ, Nam M, et al. 2020; A variant of SLC1A5 is a mitochondrial glutamine transporter for metabolic reprogramming in cancer cells. Cell Metab. 31:267–283.e12. DOI: 10.1016/j.cmet.2019.11.020. PMID: 31866442.36. Lu H, Samanta D, Xiang L, et al. 2015; Chemotherapy triggers HIF-1-dependent glutathione synthesis and copper chelation that induces the breast cancer stem cell phenotype. Proc Natl Acad Sci U S A. 112:E4600–E4609. DOI: 10.1073/pnas.1513433112. PMID: 26229077. PMCID: PMC4547233.37. Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswa-my SK, Brugge JS. 2002; The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 111:29–40. DOI: 10.1016/s0092-8674(02)01001-2. PMID: 12372298.38. Zhang K, Manninen A. 2019; 3D cell culture models of epithelial tissues. Methods Mol Biol. 1926:77–84. DOI: 10.1007/978-1-4939-9021-4_7. PMID: 30742264.39. Schafer ZT, Grassian AR, Song L, et al. 2009; Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nature. 461:109–113. DOI: 10.1038/nature08268. PMID: 19693011. PMCID: PMC2931797.40. Wiel C, Le Gal K, Ibrahim MX, et al. 2019; BACH1 stabilization by antioxidants stimulates lung cancer metastasis. Cell. 178:330–345.e22. DOI: 10.1016/j.cell.2019.06.005. PMID: 31257027.41. Hawk MA, Schafer ZT. 2018; Mechanisms of redox metabolism and cancer cell survival during extracellular matrix detachment. J Biol Chem. 293:7531–7537. DOI: 10.1074/jbc.tm117.000260. PMID: 29339552. PMCID: PMC5961063.42. Jiang L, Shestov AA, Swain P, et al. 2016; Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature. 532:255–258. DOI: 10.1038/nature17393. PMID: 27049945. PMCID: PMC4860952.43. Ubellacker JM, Tasdogan A, Ramesh V, et al. 2020; Lymph protects metastasizing melanoma cells from ferroptosis. Nature. 585:113–118. DOI: 10.1038/s41586-020-2623-z. PMID: 32814895. PMCID: PMC7484468.44. Wu M, Zhang X, Zhang W, et al. 2022; Cancer stem cell regulated phenotypic plasticity protects metastasized cancer cells from ferroptosis. Nat Commun. 13:1371. DOI: 10.3410/f.741846098.793594846. PMID: 35296660. PMCID: PMC8927306.45. Nogueira V, Hay N. 2013; Molecular pathways: reactive oxygen species homeostasis in cancer cells and implications for cancer therapy. Clin Cancer Res. 19:4309–4314. DOI: 10.1158/1078-0432.ccr-12-1424. PMID: 23719265. PMCID: PMC3933310.46. Luo M, Shang L, Brooks MD, et al. 2018; Targeting breast cancer stem cell state equilibrium through modulation of redox signaling. Cell Metab. 28:69–86.e6. DOI: 10.1016/j.cmet.2018.06.006. PMID: 29972798. PMCID: PMC6037414.47. Fortmann SP, Burda BU, Senger CA, Lin JS, Whitlock EP. 2013; Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: an updated systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 159:824–834. DOI: 10.7326/0003-4819-159-12-201312170-00729. PMID: 24217421.48. Alpha-Tocopherol. Beta Carotene Cancer Prevention Study Group. 1994; The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 330:1029–1035. DOI: 10.1056/nejm199404143301501. PMID: 8127329.49. Goodman GE, Thornquist MD, Balmes J, et al. 2004; The beta-carotene and retinol efficacy trial: incidence of lung cancer and cardiovascular disease mortality during 6-year follow-up after stopping beta-carotene and retinol supplements. J Natl Cancer Inst. 96:1743–1750. DOI: 10.1093/jnci/djh320. PMID: 15572756.50. Klein EA, Thompson IM Jr, Tangen CM, et al. 2011; Vitamin E and the risk of prostate cancer: the selenium and vitamin E cancer prevention trial (SELECT). JAMA. 306:1549–1556. DOI: 10.1016/j.yonc.2012.06.005. PMID: 21990298. PMCID: PMC4169010.51. O'Dwyer PJ, Hamilton TC, Young RC, et al. 1992; Depletion of glutathione in normal and malignant human cells in vivo by buthionine sulfoximine: clinical and biochemical results. J Natl Cancer Inst. 84:264–267. DOI: 10.1093/jnci/84.4.264. PMID: 1734088.52. Harris IS, Treloar AE, Inoue S, et al. 2015; Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell. 27:211–222. DOI: 10.1016/j.ccell.2014.11.019. PMID: 25620030.53. Harris IS, Endress JE, Coloff JL, et al. 2019; Deubiquitinases maintain protein homeostasis and survival of cancer cells upon glutathione depletion. Cell Metab. 29:1166–1181.e6. DOI: 10.1016/j.cmet.2019.01.020. PMID: 30799286. PMCID: PMC6506399.54. Ebbing M, Bønaa KH, Nygård O, et al. 2009; Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 302:2119–2126. DOI: 10.1001/jama.2009.1622. PMID: 19920236.55. Deghan Manshadi S, Ishiguro L, Sohn KJ, et al. 2014; Folic acid supplementation promotes mammary tumor progression in a rat model. PLoS One. 9:e84635. DOI: 10.1371/journal.pone.0084635. PMID: 24465421. PMCID: PMC3897399.56. Yun J, Mullarky E, Lu C, et al. 2015; Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 350:1391–1396. DOI: 10.3410/f.725917836.793511573. PMID: 26541605. PMCID: PMC4778961.57. Xu T, Liu Y, Zhao Z, et al. 2023; Ferroptosis in cancer stem cells. Pathol Res Pract. 245:154492. DOI: 10.1016/j.prp.2023.154492. PMID: 37119732.58. Tang D, Chen X, Kang R, Kroemer G. 2021; Ferroptosis: molecular mechanisms and health implications. Cell Res. 31:107–125. DOI: 10.1038/s41422-020-00441-1. PMID: 33268902. PMCID: PMC8026611.59. Kim Y, Ju H, Yoo SY, et al. 2023; Glutathione dynamics is a potential predictive and therapeutic trait for neoadjuvant chemotherapy response in bladder cancer. Cell Rep Med. 4:101224. DOI: 10.1016/j.xcrm.2023.101224. PMID: 37797616. PMCID: PMC10591055.60. Romero R, Sayin VI, Davidson SM, et al. 2017; Keap1 loss promotes KRAS-driven lung cancer and results in dependence on glutaminolysis. Nat Med. 23:1362–1368. DOI: 10.1038/nm.4407. PMID: 28967920. PMCID: PMC5677540.61. Biton M, Haber AL, Rogel N, et al. 2018; T helper cell cytokines modulate intestinal stem cell renewal and differentiation. Cell. 175:1307–1320.e22. DOI: 10.1016/j.cell.2018.10.008. PMID: 30392957. PMCID: PMC6239889.62. Bayik D, Lathia JD. 2021; Cancer stem cell-immune cell crosstalk in tumour progression. Nat Rev Cancer. 21:526–536. DOI: 10.1038/s41568-021-00366-w. PMID: 34103704. PMCID: PMC8740903.63. Chang AL, Miska J, Wainwright DA, et al. 2016; CCL2 produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res. 76:5671–5682. DOI: 10.1158/0008-5472.can-16-0144. PMID: 27530322. PMCID: PMC5050119.64. You Y, Li Y, Li M, et al. 2018; Ovarian cancer stem cells promote tumour immune privilege and invasion via CCL5 and regulatory T cells. Clin Exp Immunol. 191:60–73. DOI: 10.1111/cei.13044. PMID: 28868628. PMCID: PMC5721255.65. Ban Y, Mai J, Li X, et al. 2017; Targeting autocrine CCL5-CCR5 axis reprograms immunosuppressive myeloid cells and reinvigorates antitumor immunity. Cancer Res. 77:2857–2868. DOI: 10.1158/0008-5472.CAN-16-2913. PMID: 28416485. PMCID: PMC5484057.66. Nakano M, Kikushige Y, Miyawaki K, et al. 2019; Dedifferentia-tion process driven by TGF-beta signaling enhances stem cell properties in human colorectal cancer. Oncogene. 38:780–793. DOI: 10.1038/s41388-018-0480-0. PMID: 30181548.67. Wainwright DA, Balyasnikova IV, Chang AL, et al. 2012; IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res. 18:6110–6121. DOI: 10.1158/1078-0432.ccr-12-2130. PMID: 22932670. PMCID: PMC3500434.68. Wang D, Fu L, Sun H, Guo L, DuBois RN. 2015; Prostaglandin E2 promotes colorectal cancer stem cell expansion and metastasis in mice. Gastroenterology. 149:1884–1895.e4. DOI: 10.1053/j.gastro.2015.07.064. PMID: 26261008. PMCID: PMC4762503.69. Facciabene A, Peng X, Hagemann IS, et al. 2011; Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature. 475:226–230. DOI: 10.3410/f.12919956.14210054. PMID: 21753853.70. Zhang Y, Zoltan M, Riquelme E, et al. 2018; Immune cell production of interleukin 17 induces stem cell features of pancreatic intraepithelial neoplasia cells. Gastroenterology. 155:210–223.e3. DOI: 10.1053/j.gastro.2018.03.041. PMID: 29604293. PMCID: PMC6035075.71. He W, Wu J, Shi J, et al. 2018; IL22RA1/STAT3 signaling promotes stemness and tumorigenicity in pancreatic cancer. Cancer Res. 78:3293–3305. DOI: 10.1158/0008-5472.CAN-17-3131. PMID: 29572224.72. Ben-Porath I, Thomson MW, Carey VJ, et al. 2008; An embryonic stem cell-like gene expression signature in poorly differentiated aggressive human tumors. Nat Genet. 40:499–507. DOI: 10.1038/ng.127. PMID: 18443585. PMCID: PMC2912221.73. Yang S, Wang B, Guan C, et al. 2011; Foxp3+IL-17+ T cells promote development of cancer-initiating cells in colorectal cancer. J Leukoc Biol. 89:85–91.74. Tsuchiya H, Shiota G. 2021; Immune evasion by cancer stem cells. Regen Ther. 17:20–33. DOI: 10.1016/j.reth.2021.02.006. PMID: 33778133. PMCID: PMC7966825.75. Zheng F, Dang J, Zhang H, et al. 2018; Cancer stem cell vaccination with PD-L1 and CTLA-4 blockades enhances the eradication of melanoma stem cells in a mouse tumor model. J Immunother. 41:361–368. DOI: 10.1097/cji.0000000000000242. PMID: 30063587. PMCID: PMC6128768.76. Chen Y, Li M, Cao J, et al. 2021; CTLA-4 promotes lymphoma progression through tumor stem cell enrichment and immunosuppression. Open Life Sci. 16:909–919. DOI: 10.1515/biol-2021-0094. PMID: 34553071. PMCID: PMC8422981.77. Deshmukh AP, den Hollander P, Kuburich NA, Vasaikar S, Joseph R, Mani SA. 2022; Enrichment of cancer stem cells in a tumorsphere assay. Methods Mol Biol. 2429:501–507. DOI: 10.1007/978-1-0716-1979-7_34. PMID: 35507184.78. Case AJ, McGill JL, Tygrett LT, et al. 2011; Elevated mitochondrial superoxide disrupts normal T cell development, impairing adaptive immune responses to an influenza challenge. Free Radic Biol Med. 50:448–458. DOI: 10.1016/j.freeradbiomed.2010.11.025. PMID: 21130157. PMCID: PMC3026081.79. Tse HM, Thayer TC, Steele C, et al. 2010; NADPH oxidase deficiency regulates Th lineage commitment and modulates autoimmunity. J Immunol. 185:5247–5258. DOI: 10.4049/jimmunol.1001472. PMID: 20881184. PMCID: PMC3190397.80. Hanschmann EM, Godoy JR, Berndt C, Hudemann C, Lillig CH. 2013; Thioredoxins, glutaredoxins, and peroxiredoxins--molecular mechanisms and health significance: from cofactors to antioxidants to redox signaling. Antioxid Redox Signal. 19:1539–1605. DOI: 10.1089/ars.2012.4599. PMID: 23397885. PMCID: PMC3797455.81. Marino SM, Gladyshev VN. 2010; Cysteine function governs its conservation and degeneration and restricts its utilization on protein surfaces. J Mol Biol. 404:902–916. DOI: 10.1016/j.jmb.2010.09.027. PMID: 20950627. PMCID: PMC3061813.82. Gmünder H, Eck HP, Benninghoff B, Roth S, Dröge W. 1990; Macrophages regulate intracellular glutathione levels of lymphocytes. Evidence for an immunoregulatory role of cysteine. Cell Immunol. 129:32–46. DOI: 10.1016/0008-8749(90)90184-s. PMID: 2364441.83. Angelini G, Gardella S, Ardy M, et al. 2002; Antigen-presenting dendritic cells provide the reducing extracellular microenvironment required for T lymphocyte activation. Proc Natl Acad Sci U S A. 99:1491–1496. DOI: 10.1073/pnas.022630299. PMID: 11792859. PMCID: PMC122218.84. Yan Z, Garg SK, Kipnis J, Banerjee R. 2009; Extracellular redox modulation by regulatory T cells. Nat Chem Biol. 5:721–723. DOI: 10.1038/nchembio.212. PMID: 19718041. PMCID: PMC2760945.85. Yan Z, Garg SK, Banerjee R. 2010; Regulatory T cells interfere with glutathione metabolism in dendritic cells and T cells. J Biol Chem. 285:41525–41532. DOI: 10.1074/jbc.m110.189944. PMID: 21037289. PMCID: PMC3009879.86. Lee K, Won HY, Bae MA, Hong JH, Hwang ES. 2011; Spontaneous and aging-dependent development of arthritis in NADPH oxidase 2 deficiency through altered differentiation of CD11b+ and Th/Treg cells. Proc Natl Acad Sci U S A. 108:9548–9553.87. Noel S, Martina MN, Bandapalle S, et al. 2015; T lymphocyte-specific activation of Nrf2 protects from AKI. J Am Soc Nephrol. 26:2989–3000. DOI: 10.1681/ASN.2014100978. PMID: 26293820. PMCID: PMC4657838.88. Zhang D, Jin W, Wu R, et al. 2019; High glucose intake exacerbates autoimmunity through reactive-oxygen-species-mediated TGF-β cytokine activation. Immunity. 51:671–681.e5. DOI: 10.1016/j.immuni.2019.08.001. PMID: 31451397. PMCID: PMC9811990.89. Mak TW, Grusdat M, Duncan GS, et al. 2017; Glutathione primes T cell metabolism for inflammation. Immunity. 46:1089–1090. DOI: 10.1016/j.immuni.2017.06.009. PMID: 28423341.90. Roychoudhuri R, Hirahara K, Mousavi K, et al. 2013; BACH2 represses effector programs to stabilize T(reg)-mediated immune homeostasis. Nature. 498:506–510. DOI: 10.3410/f.718015787.793480628. PMID: 23728300. PMCID: PMC3710737.91. Yu X, Lao Y, Teng XL, et al. 2018; SENP3 maintains the stability and function of regulatory T cells via BACH2 deSUMOylation. Nat Commun. 9:3157. DOI: 10.1038/s41467-018-05676-6. PMID: 30089837. PMCID: PMC6082899.92. Yeh ET, Gong L, Kamitani T. 2000; Ubiquitin-like proteins: new wines in new bottles. Gene. 248:1–14. DOI: 10.1016/s0378-1119(00)00139-6. PMID: 10806345.93. Flotho A, Melchior F. 2013; Sumoylation: a regulatory protein modification in health and disease. Annu Rev Biochem. 82:357–385. DOI: 10.1146/annurev-biochem-061909-093311. PMID: 23746258.94. Hang S, Paik D, Yao L, et al. 2019; Bile acid metabolites control TH17 and Treg cell differentiation. Nature. 576:143–148. DOI: 10.1038/s41586-019-1785-z. PMID: 31776512. PMCID: PMC6949019.95. Alissafi T, Kalafati L, Lazari M, et al. 2020; Mitochondrial oxidative damage underlies regulatory T cell defects in autoi-mmunity. Cell Metab. 32:591–604.e7. DOI: 10.1016/j.cmet.2020.07.001. PMID: 32738205. PMCID: PMC7611060.96. Heo J, Noh BJ, Lee S, et al. 2020; Phosphorylation of TFCP2L1 by CDK1 is required for stem cell pluripotency and bladder carcinogenesis. EMBO Mol Med. 12:e10880. DOI: 10.15252/emmm.201910880. PMID: 31709755. PMCID: PMC6949511.97. Heo J, Lee J, Nam YJ, et al. 2022; The CDK1/TFCP2L1/ID2 cascade offers a novel combination therapy strategy in a preclinical model of bladder cancer. Exp Mol Med. 54:801–811. DOI: 10.1038/s12276-022-00786-0. PMID: 35729325. PMCID: PMC9256744.98. Oh Y, Jung HR, Min S, et al. 2021; Targeting antioxidant enzymes enhances the therapeutic efficacy of the BCL-XL inhibitor ABT-263 in KRAS-mutant colorectal cancers. Can-cer Lett. 497:123–136. DOI: 10.1016/j.canlet.2020.10.018. PMID: 33068701.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prospects for Manipulation of Mesenchymal Stem Cells in Tumor Therapy: Anti-Angiogenesis Property on the Spotlight
- New Findings on Breast Cancer Stem Cells: A Review
- Cancer Stem Cells in Brain Tumors and Their Lineage Hierarchy
- Tumor Cells and Cancer-Associated Fibroblasts: A Synergistic Crosstalk to Promote Thyroid Cancer
- Interactions between Immune Cells and Tumor Cells