Endocrinol Metab.
2020 Dec;35(4):673-680. 10.3803/EnM.2020.401.
Tumor Cells and Cancer-Associated Fibroblasts: A Synergistic Crosstalk to Promote Thyroid Cancer
- Affiliations
-
- 1Center for Research in Clinical Biochemistry and Immunology (CIBICI)-National Scientific and Technical Research Council (CONICET), School of Chemical Sciences, National University of Córdoba, Cordoba, Argentina
- 2Laboratory of Molecular Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA
- KMID: 2510996
- DOI: http://doi.org/10.3803/EnM.2020.401
Abstract
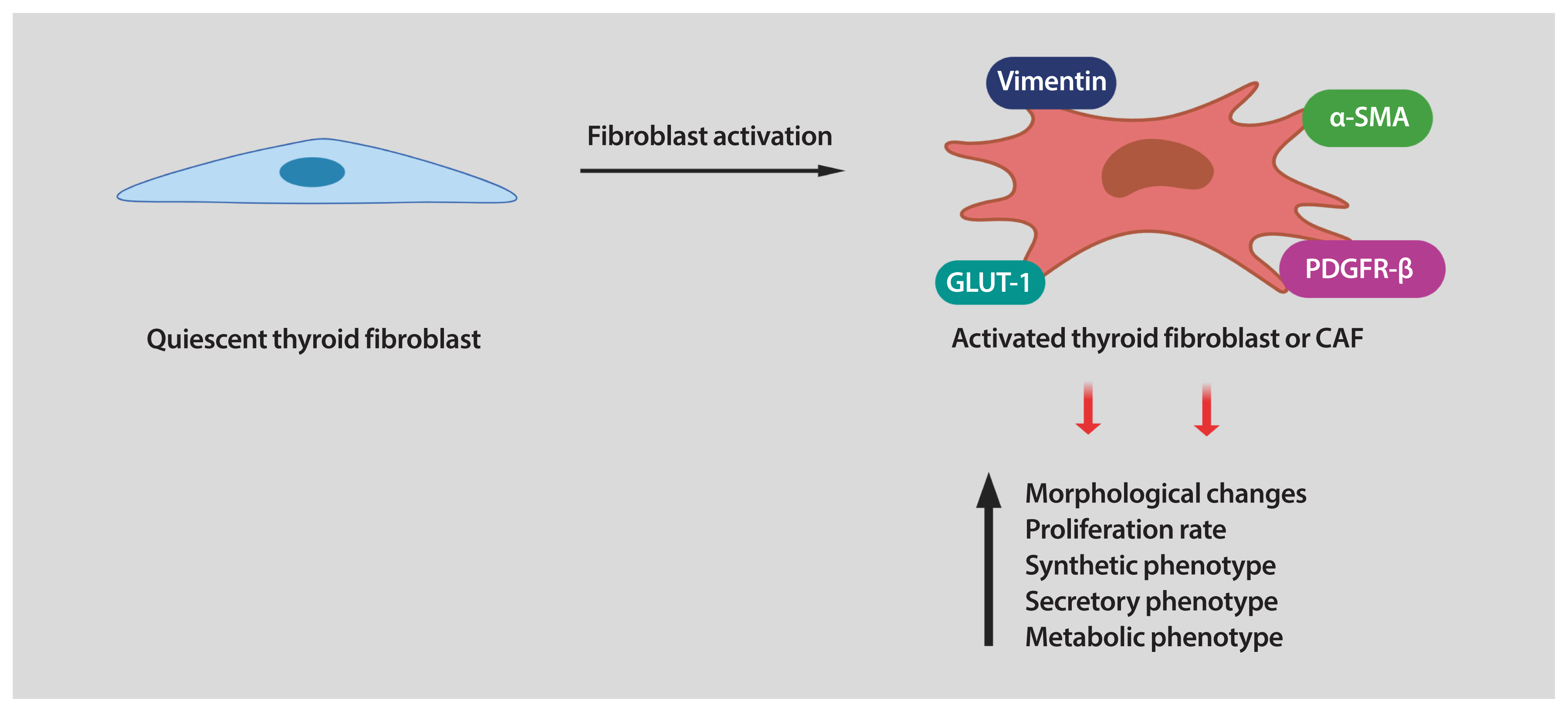
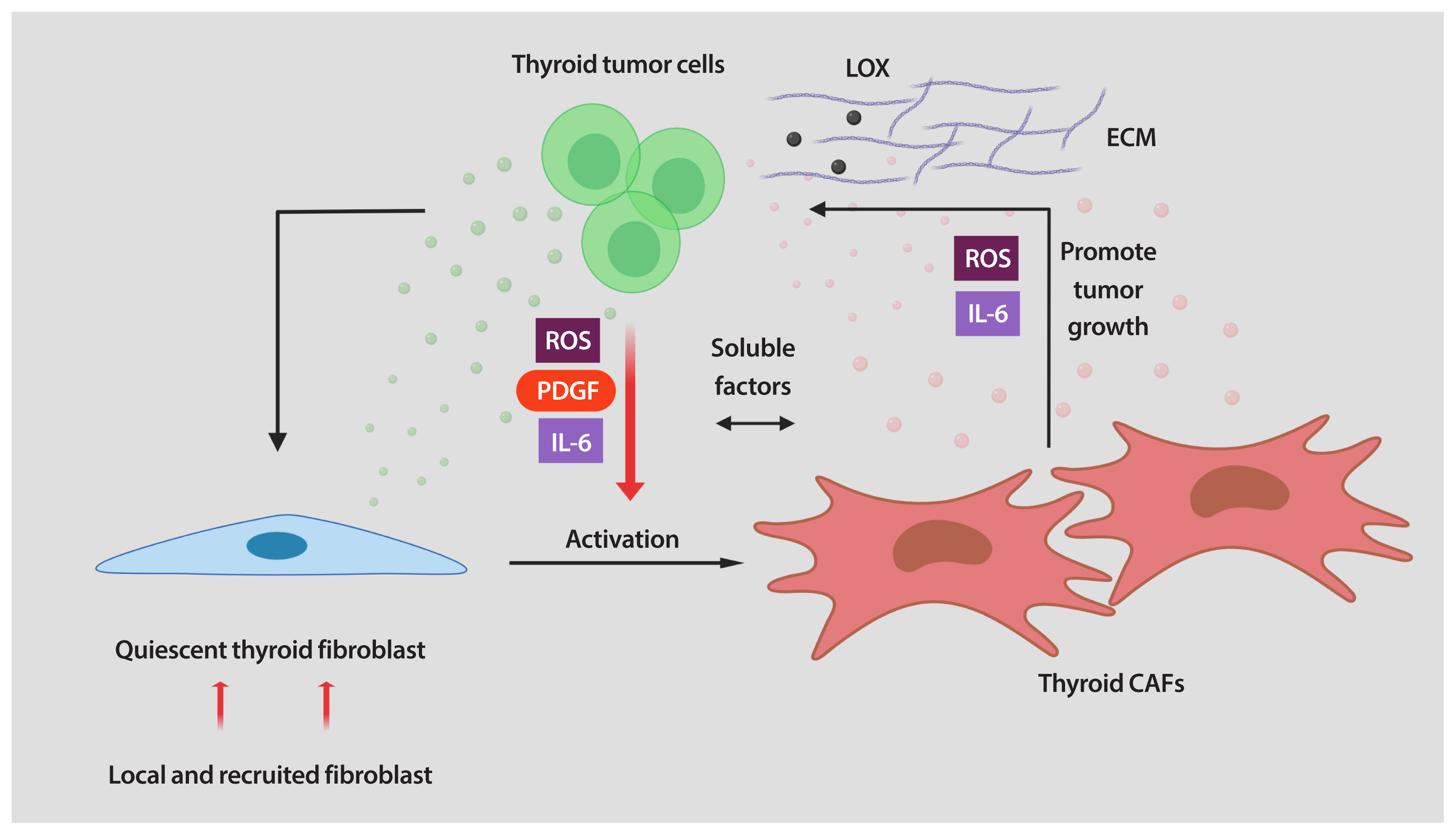
- Thyroid cancer is the most common endocrine malignancy. Although most thyroid cancer patients are successfully treated and have an excellent prognosis, a percentage of these patients will develop aggressive disease and, eventually, progress to anaplastic thyroid cancer. Since most patients with this type of aggressive thyroid carcinoma will die from the disease, new treatment strategies are urgently needed. Tumor cells live in a complex and dynamic tumor microenvironment composed of different types of stromal cells. Cancer-associated fibroblasts (CAFs) are one of the most important cell components in the tumor microenvironment of most solid tumors, including thyroid cancer. CAFs originate mainly from mesenchymal cells and resident fibroblasts that are activated and reprogrammed in response to paracrine factors and cytokines produced and released by tumor cells. Upon reprogramming, which is distinguished by the expression of different marker proteins, CAFs synthesize and secret soluble factors. The secretome of CAFs directly impacts different functions of tumor cells. This bi-directional interplay between CAFs and tumor cells within the tumor microenvironment ends up fostering tumor cancer progression. CAFs are therefore key regulators of tumor progression and represent an under-explored therapeutic target in thyroid cancer.
Keyword
Figure
Reference
-
1. National Cancer Institute. SEER cancer stat facts: thyroid cancer [Internet]. Bethesda: NCI;2020. [cited 2020 Oct 14]. Available from: https://seer.cancer.gov/statfacts/html/thyro.html.2. Davies L, Welch HG. Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg. 2014; 140:317–22.
Article3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020; 70:7–30.
Article4. Fagin JA, Wells SA Jr. Biologic and clinical perspectives on thyroid cancer. N Engl J Med. 2016; 375:1054–67.
Article5. Cabanillas ME, McFadden DG, Durante C. Thyroid cancer. Lancet. 2016; 388:2783–95.
Article6. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144:646–74.
Article7. Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012; 21:309–22.
Article8. Cirri P, Chiarugi P. Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res. 2011; 1:482–97.9. Ohlund D, Elyada E, Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med. 2014; 211:1503–23.
Article10. Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016; 16:582–98.
Article11. Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020; 20:174–86.
Article12. Virchow R. Die Cellularpathologie in lhrer Begruendung auf Physiologische und Pathologische Gewebelehre. Berlin: Hirschwald;1858.13. Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006; 6:392–401.
Article14. Mueller MM, Fusenig NE. Friends or foes: bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004; 4:839–49.
Article15. Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995; 146:56–66.16. Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002; 3:349–63.
Article17. Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986; 315:1650–9.18. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014; 159:676–90.19. Suzuki H, Willingham MC, Cheng SY. Mice with a mutation in the thyroid hormone receptor beta gene spontaneously develop thyroid carcinoma: a mouse model of thyroid carcinogenesis. Thyroid. 2002; 12:963–9.
Article20. Cheng SY, Leonard JL, Davis PJ. Molecular aspects of thyroid hormone actions. Endocr Rev. 2010; 31:139–70.
Article21. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016; 126:1052–66.
Article22. Molinaro E, Romei C, Biagini A, Sabini E, Agate L, Mazzeo S, et al. Anaplastic thyroid carcinoma: from clinicopathology to genetics and advanced therapies. Nat Rev Endocrinol. 2017; 13:644–60.
Article23. Xu B, Fuchs T, Dogan S, Landa I, Katabi N, Fagin JA, et al. Dissecting anaplastic thyroid carcinoma: a comprehensive clinical, histologic, immunophenotypic, and molecular study of 360 cases. Thyroid. 2020; 30:1505–17.
Article24. Ceolin L, Duval MADS, Benini AF, Ferreira CV, Maia AL. Medullary thyroid carcinoma beyond surgery: advances, challenges, and perspectives. Endocr Relat Cancer. 2019; 26:R499–518.
Article25. Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011; 7:569–80.
Article26. Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer. 2013; 13:184–99.
Article27. Pozdeyev N, Gay LM, Sokol ES, Hartmaier R, Deaver KE, Davis S, et al. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clin Cancer Res. 2018; 24:3059–68.
Article28. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133.
Article29. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000; 100:57–70.
Article30. Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013; 19:1423–37.
Article31. Schnittert J, Bansal R, Prakash J. Targeting pancreatic stellate cells in cancer. Trends Cancer. 2019; 5:128–42.
Article32. LeBleu VS, Kalluri R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. Dis Model Mech. 2018; 11:dmm029447.
Article33. Cho JG, Byeon HK, Oh KH, Baek SK, Kwon SY, Jung KY, et al. Clinicopathological significance of cancer-associated fibroblasts in papillary thyroid carcinoma: a predictive marker of cervical lymph node metastasis. Eur Arch Otorhinolaryngol. 2018; 275:2355–61.
Article34. Sun WY, Jung WH, Koo JS. Expression of cancer-associated fibroblast-related proteins in thyroid papillary carcinoma. Tumour Biol. 2016; 37:8197–207.
Article35. Minna E, Brich S, Todoerti K, Pilotti S, Collini P, Bonaldi E, et al. Cancer associated fibroblasts and senescent thyroid cells in the invasive front of thyroid carcinoma. Cancers (Basel). 2020; 12:112.
Article36. Caillou B, Talbot M, Weyemi U, Pioche-Durieu C, Al Ghuzlan A, Bidart JM, et al. Tumor-associated macrophages (TAMs) form an interconnected cellular supportive network in anaplastic thyroid carcinoma. PLoS One. 2011; 6:e22567.
Article37. Ryder M, Gild M, Hohl TM, Pamer E, Knauf J, Ghossein R, et al. Genetic and pharmacological targeting of CSF-1/CSF-1R inhibits tumor-associated macrophages and impairs BRAF-induced thyroid cancer progression. PLoS One. 2013; 8:e54302.
Article38. Jolly LA, Novitskiy S, Owens P, Massoll N, Cheng N, Fang W, et al. Fibroblast-mediated collagen remodeling within the tumor microenvironment facilitates progression of thyroid cancers driven by BrafV600E and Pten loss. Cancer Res. 2016; 76:1804–13.
Article39. Zhang J, Wang Y, Li D, Jing S. Notch and TGF-β/Smad3 pathways are involved in the interaction between cancer cells and cancer-associated fibroblasts in papillary thyroid carcinoma. Tumour Biol. 2014; 35:379–85.
Article40. Fozzatti L, Alamino VA, Park S, Giusiano L, Volpini X, Zhao L, et al. Interplay of fibroblasts with anaplastic tumor cells promotes follicular thyroid cancer progression. Sci Rep. 2019; 9:8028.
Article41. Saitoh O, Mitsutake N, Nakayama T, Nagayama Y. Fibroblast-mediated in vivo and in vitro growth promotion of tumorigenic rat thyroid carcinoma cells but not normal Fisher rat thyroid follicular cells. Thyroid. 2009; 19:735–42.
Article42. Ricard-Blum S. The collagen family. Cold Spring Harb Perspect Biol. 2011; 3:a004978.
Article43. Tenti P, Vannucci L. Lysyl oxidases: linking structures and immunity in the tumor microenvironment. Cancer Immunol Immunother. 2020; 69:223–35.
Article44. Boufraqech M, Nilubol N, Zhang L, Gara SK, Sadowski SM, Mehta A, et al. miR30a inhibits LOX expression and anaplastic thyroid cancer progression. Cancer Res. 2015; 75:367–77.
Article45. Boufraqech M, Patel D, Nilubol N, Powers A, King T, Shell J, et al. Lysyl oxidase is a key player in BRAF/MAPK pathway-driven thyroid cancer aggressiveness. Thyroid. 2019; 29:79–92.
Article46. Chen X, Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019; 18:99–115.
Article47. Melisi D, Garcia-Carbonero R, Macarulla T, Pezet D, Deplanque G, Fuchs M, et al. TGFβ receptor inhibitor galunisertib is linked to inflammation- and remodeling-related proteins in patients with pancreatic cancer. Cancer Chemother Pharmacol. 2019; 83:975–91.
Article48. Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018; 15:234–48.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interactions between Immune Cells and Tumor Cells
- Tamoxifen Resistance and Crosstalk of Signal Transduction in Breast Cancer
- New Strategies for Combined Radioiodine Therapy in Refractory Thyroid Cancer
- A Case of Trophoblastic Tumor Associated with Papillary Thyroid Cancer
- The crosstalk of Wnt/β-catenin signaling and p53 in acute kidney injury and chronic kidney disease