Korean J Ophthalmol.
2016 Feb;30(1):66-75. 10.3341/kjo.2016.30.1.66.
Effect of Nitric Oxide on the Expression of Matrix Metalloproteinase and Its Association with Migration of Cultured Trabecular Meshwork Cells
- Affiliations
-
- 1Department of Ophthalmology, Catholic University of Daegu School of Medicine, Daegu, Korea. jwkim@cu.ac.kr
- KMID: 2363893
- DOI: http://doi.org/10.3341/kjo.2016.30.1.66
Abstract
- PURPOSE
To determine the effect of exogenous nitric oxide (NO) on the migration of trabecular meshwork (TM) cells and its association with expression of matrix metalloproteinases (MMPs).
METHODS
Primary human TM cells treated with 1 or 10 microM S-nitroso-N-acetyl-penicillamine (SNAP) and examined for changes in adherence. TM cells were seeded onto transwell culture inserts, and changes in their migratory activity were quantified. Reverse transcription polymerase chain reaction was performed to determine the relative changes in mRNA expression of MMPs and tissue inhibitor of metalloproteinases (TIMPs).
RESULTS
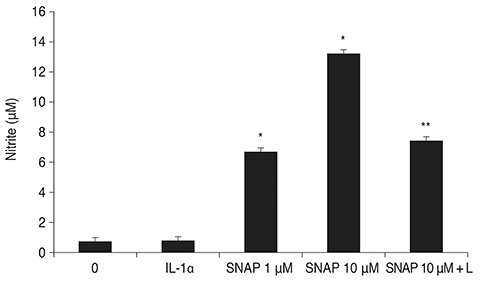
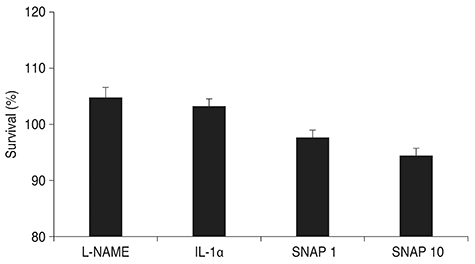
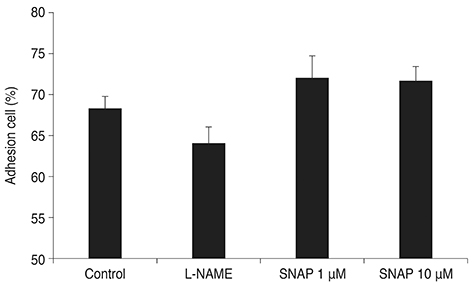
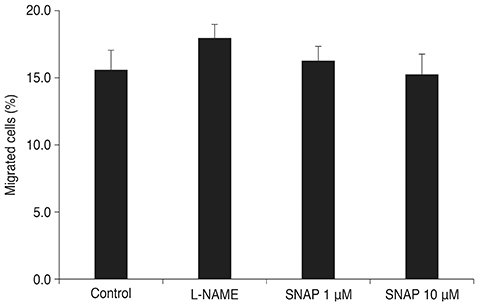
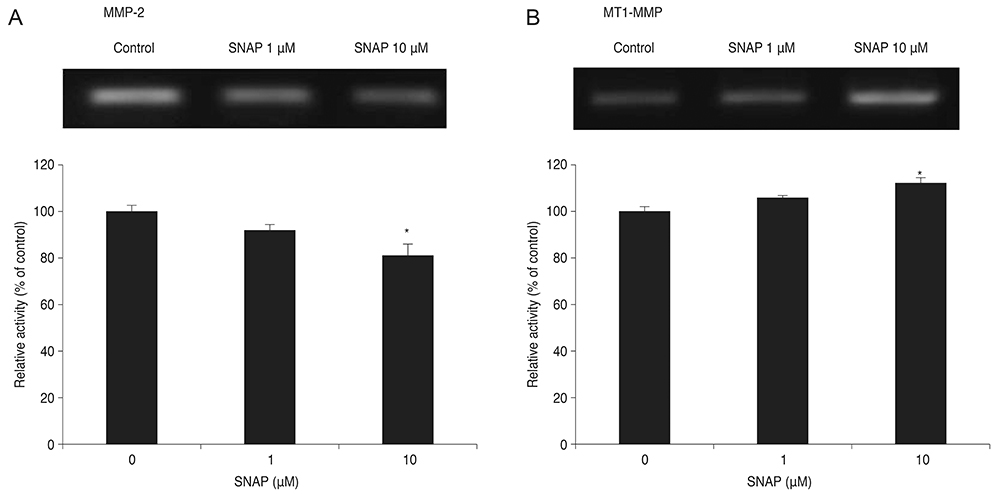
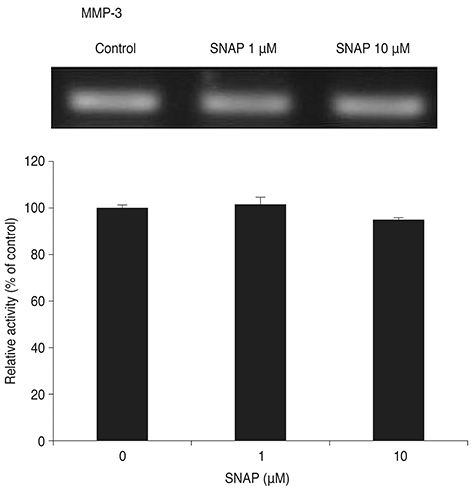
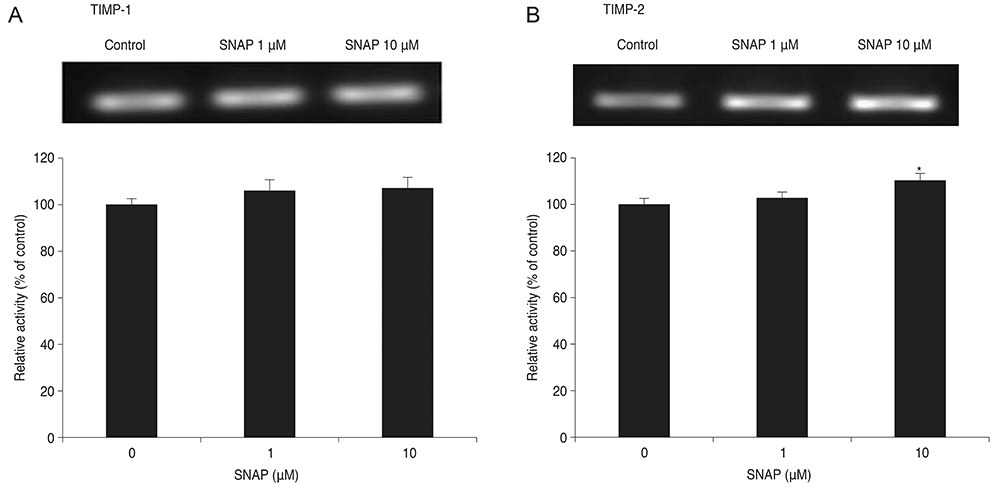
Treatment with SNAP did not significantly suppress TM cell adhesion or migration (p > 0.05). Treatment of TM cells with 10 microM SNAP decreased expression of MMP-2 and increased expression of membrane type MMP-1 and TIMP-2. Treatment with interleukin-1alpha triggered MMP-3 expression but did not exert significant effects on MMP-3 activation in response to SNAP.
CONCLUSIONS
These data suggest that NO revealed no significant effect on the migration of TM cells because NO decreased MMP-2 and increased TIMP-2 expression. Although expression of certain MMPs and TIMPs change in response to NO donors, NO may modulate trabecular outflow by changing the cellular production of extracellular matrix without having a significant effect on the migration of TM cells.
MeSH Terms
-
Cell Movement/*drug effects
Cell Survival/drug effects
Cells, Cultured
DNA Primers/chemistry
Gene Expression Regulation, Enzymologic/*physiology
Humans
Matrix Metalloproteinases/*genetics
Nitric Oxide Donors/*pharmacology
RNA, Messenger/genetics
Real-Time Polymerase Chain Reaction
S-Nitroso-N-Acetylpenicillamine/*pharmacology
Tissue Inhibitor of Metalloproteinase-2/*genetics
Trabecular Meshwork/cytology/*drug effects/enzymology
DNA Primers
Matrix Metalloproteinases
Nitric Oxide Donors
RNA, Messenger
S-Nitroso-N-Acetylpenicillamine
Tissue Inhibitor of Metalloproteinase-2
Figure
Cited by 1 articles
-
Effect and Mechanism of Phosphodiesterase Inhibitors on Trabecular Outflow
Jae Woo Kim, Jong Been Lee, So Hyung Lee
Korean J Ophthalmol. 2019;33(5):414-421. doi: 10.3341/kjo.2019.0057.
Reference
-
1. Gasiorowski JZ, Russell P. Biological properties of trabecular meshwork cells. Exp Eye Res. 2009; 88:671–675.2. Bradley JM, Kelley MJ, Zhu X, et al. Effects of mechanical stretching on trabecular matrix metalloproteinases. Invest Ophthalmol Vis Sci. 2001; 42:1505–1513.3. Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp Eye Res. 2008; 86:543–561.4. Alexander JP, Samples JR, Van Buskirk EM, Acott TS. Expression of matrix metalloproteinases and inhibitor by human trabecular meshwork. Invest Ophthalmol Vis Sci. 1991; 32:172–180.5. Borras T. Gene expression in the trabecular meshwork and the influence of intraocular pressure. Prog Retin Eye Res. 2003; 22:435–463.6. Fuchshofer R, Welge-Lussen U, Lutjen-Drecoll E. The effect of TGF-beta2 on human trabecular meshwork extracellular proteolytic system. Exp Eye Res. 2003; 77:757–765.7. Lo WR, Rowlette LL, Caballero M, et al. Tissue differential microarray analysis of dexamethasone induction reveals potential mechanisms of steroid glaucoma. Invest Ophthalmol Vis Sci. 2003; 44:473–485.8. Vittal V, Rose A, Gregory KE, et al. Changes in gene expression by trabecular meshwork cells in response to mechanical stretching. Invest Ophthalmol Vis Sci. 2005; 46:2857–2868.9. Oh DJ, Martin JL, Williams AJ, et al. Effect of latanoprost on the expression of matrix metalloproteinases and their tissue inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006; 47:3887–3895.10. Bradley JM, Vranka J, Colvis CM, et al. Effect of matrix metalloproteinases activity on outflow in perfused human organ culture. Invest Ophthalmol Vis Sci. 1998; 39:2649–2658.11. Grierson I, Hogg P. The proliferative and migratory activities of trabecular meshwork cells. Prog Retin Eye Res. 1995; 15:33–67.12. Alvarado J, Murphy C, Polansky J, Juster R. Age-related changes in trabecular meshwork cellularity. Invest Ophthalmol Vis Sci. 1981; 21:714–727.13. Grierson I, Howes RC. Age-related depletion of the cell population in the human trabecular meshwork. Eye (Lond). 1987; 1(Pt 2):204–210.14. Grierson I, Wang Q, McMenamin PG, Lee WR. The effects of age and antiglaucoma drugs on the meshwork cell population. Res Clin Forums. 1981; 4:69–92.15. Rubanyi GM. The role of endothelium in cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol. 1993; 22 Suppl 4:S1–S14.16. Jeremy JY, Rowe D, Emsley AM, Newby AC. Nitric oxide and the proliferation of vascular smooth muscle cells. Cardiovasc Res. 1999; 43:580–594.17. Newby AC, George SJ. Proliferation, migration, matrix turnover, and death of smooth muscle cells in native coronary and vein graft atherosclerosis. Curr Opin Cardiol. 1996; 11:574–582.18. Trachtman H, Futterweit S, Garg P, et al. Nitric oxide stimulates the activity of a 72-kDa neutral matrix metalloproteinase in cultured rat mesangial cells. Biochem Biophys Res Commun. 1996; 218:704–708.19. Murohara T, Witzenbichler B, Spyridopoulos I, et al. Role of endothelial nitric oxide synthase in endothelial cell migration. Arterioscler Thromb Vasc Biol. 1999; 19:1156–1161.20. Ziche M, Morbidelli L, Masini E, et al. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest. 1994; 94:2036–2044.21. Polansky JR, Weinreb RN, Baxter JD, Alvarado J. Human trabecular cells .I. Establishment in tissue culture and growth characteristics. Invest Ophthalmol Vis Sci. 1979; 18:1043–1049.22. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65:55–63.23. Green LC, Wagner DA, Glogowski J, et al. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982; 126:131–138.24. Okayama N, Ichikawa H, Coe L, et al. Exogenous NO enhances hydrogen peroxide-mediated neutrophil adherence to cultured endothelial cells. Am J Physiol. 1998; 274(5 Pt 1):L820–L826.25. Okouchi M, Okayama N, Shimizu M, et al. High insulin exacerbates neutrophil-endothelial cell adhesion through endothelial surface expression of intercellular adhesion molecule-1 via activation of protein kinase C and mitogen-activated protein kinase. Diabetologia. 2002; 45:556–559.26. Hogg P, Calthorpe M, Batterbury M, Grierson I. Aqueous humor stimulates the migration of human trabecular meshwork cells in vitro. Invest Ophthalmol Vis Sci. 2000; 41:1091–1098.27. Sato H, Kinoshita T, Takino T, et al. Activation of a recombinant membrane type 1-matrix metalloproteinase (MT1-MMP) by furin and its interaction with tissue inhibitor of metalloproteinases (TIMP)-2. FEBS Lett. 1996; 393:101–104.28. Oh DJ, Martin JL, Williams AJ, et al. Analysis of expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human ciliary body after latanoprost. Invest Ophthalmol Vis Sci. 2006; 47:953–963.29. Maulik N, Engelman DT, Watanabe M, et al. Nitric oxide/carbon monoxide: a molecular switch for myocardial preservation during ischemia. Circulation. 1996; 94:9 Suppl. II398–II406.30. Eberhardt W, Beeg T, Beck KF, et al. Nitric oxide modulates expression of matrix metalloproteinase-9 in rat mesangial cells. Kidney Int. 2000; 57:59–69.31. Zhang HJ, Zhao W, Venkataraman S, et al. Activation of matrix metalloproteinase-2 by overexpression of manganese superoxide dismutase in human breast cancer MCF-7 cells involves reactive oxygen species. J Biol Chem. 2002; 277:20919–20926.32. Upchurch GR Jr, Ford JW, Weiss SJ, et al. Nitric oxide inhibition increases matrix metalloproteinase-9 expression by rat aortic smooth muscle cells in vitro. J Vasc Surg. 2001; 34:76–83.33. Newby AC. Matrix metalloproteinases regulate migration, proliferation, and death of vascular smooth muscle cells by degrading matrix and non-matrix substrates. Cardiovasc Res. 2006; 69:614–624.34. Chen HH, Wang DL. Nitric oxide inhibits matrix metalloproteinase-2 expression via the induction of activating transcription factor 3 in endothelial cells. Mol Pharmacol. 2004; 65:1130–1140.35. Sarkar R, Meinberg EG, Stanley JC, et al. Nitric oxide reversibly inhibits the migration of cultured vascular smooth muscle cells. Circ Res. 1996; 78:225–230.36. Kawasaki K, Smith RS Jr, Hsieh CM, et al. Activation of the phosphatidylinositol 3-kinase/protein kinase Akt pathway mediates nitric oxide-induced endothelial cell migration and angiogenesis. Mol Cell Biol. 2003; 23:5726–5737.37. Lau YT, Ma WC. Nitric oxide inhibits migration of cultured endothelial cells. Biochem Biophys Res Commun. 1996; 221:670–674.38. Kook H, Ahn KY, Lee SE, et al. Nitric oxide-dependent cytoskeletal changes and inhibition of endothelial cell migration contribute to the suppression of angiogenesis by RAD50 gene transfer. FEBS Lett. 2003; 553:56–62.39. Matsuo T. Basal nitric oxide production is enhanced by hydraulic pressure in cultured human trabecular cells. Br J Ophthalmol. 2000; 84:631–635.40. Haefliger IO, Dettmann E, Liu R. Potential role of nitric oxide and endothelin in the pathogenesis of glaucoma. Surv Ophthalmol. 1999; 58:99–105.41. Schuman JS, Erickson K, Nathanson JA. Nitrovasodilator effects on intraocular pressure and outflow facility in monkeys. Exp Eye Res. 1994; 58:99–105.42. Pfeilschifter J, Eberhardt W, Huwiler A. Nitric oxide and mechanisms of redox signalling: matrix and matrix-metabolizing enzymes as prime nitric oxide targets. Eur J Pharmacol. 2001; 429:279–286.43. Kim JW, Heo H, Lee HW. Effect of nitric oxide on the proliferation of cultured porcine trabecular meshwork cells. Korean J Ophthalmol. 2003; 17:1–6.44. Noiri E, Peresleni T, Srivastava N, et al. Nitric oxide is necessary for a switch from stationary to locomoting phenotype in epithelial cells. Am J Physiol. 1996; 270(3 Pt 1):C794–C802.45. Beauvais F, Michel L, Dubertret L. Exogenous nitric oxide elicits chemotaxis of neutrophils in vitro. J Cell Physiol. 1995; 165:610–614.46. Noiri E, Lee E, Testa J, et al. Podokinesis in endothelial cell migration: role of nitric oxide. Am J Physiol. 1998; 274(1 Pt 1):C236–C244.47. Noiri E, Hu Y, Bahou WF, et al. Permissive role of nitric oxide in endothelin-induced migration of endothelial cells. J Biol Chem. 1997; 272:1747–1752.48. Genis L, Gonzalo P, Tutor AS, et al. Functional interplay between endothelial nitric oxide synthase and membrane type 1 matrix metalloproteinase in migrating endothelial cells. Blood. 2007; 110:2916–2923.49. Samples JR, Alexander JP, Acott TS. Regulation of the levels of human trabecular matrix metalloproteinases and inhibitor by interleukin-1 and dexamethasone. Invest Ophthalmol Vis Sci. 1993; 34:3386–3395.50. Pang IH, Hellberg PE, Fleenor DL, et al. Expression of matrix metalloproteinases and their inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2003; 44:3485–3493.51. Ailenberg M, Silverman M. Cellular activation of mesangial gelatinase A by cytochalasin D is accompanied by enhanced mRNA expression of both gelatinase A and its membrane-associated gelatinase A activator (MT-MMP). Biochem J. 1996; 313(Pt 3):879–884.52. Ronkko S, Rekonen P, Kaarniranta K, et al. Matrix metalloproteinases and their inhibitors in the chamber angle of normal eyes and patients with primary open-angle glaucoma and exfoliation glaucoma. Graefes Arch Clin Exp Ophthalmol. 2007; 245:697–704.53. Keller KE, Aga M, Bradley JM, et al. Extracellular matrix turnover and outflow resistance. Exp Eye Res. 2009; 88:676–682.54. Schlotzer-Schrehardt U, Lommatzsch J, Kuchle M, et al. Matrix metalloproteinases and their inhibitors in aqueous humor of patients with pseudoexfoliation syndrome/glaucoma and primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2003; 44:1117–1125.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Nitric Oxide on Adhesion and Migration of Trabecular Meshwork Cells
- Effect of Mitomycin C on the Proliferation and Nitric Oxide Production in the Cultured Trabecular Meshwork Cells
- Effect of beta-adrenergics on the Survival and Production of Nitric Oxide in the Cultured Trabecular Meshwork Cells
- Effect of Erythropoietin on the Production of Nitric Oxide in Trabecular Meshwork Cells
- Comparative Study of the Effects of Trabecular Meshwork Outflow Drugs on the Permeability and Nitric Oxide Production in Trabecular Meshwork Cells