Investig Clin Urol.
2016 Mar;57(2):106-112. 10.4111/icu.2016.57.2.106.
Value of urinary topoisomerase-IIA cell-free DNA for diagnosis of bladder cancer
- Affiliations
-
- 1Department of Urology, Chungbuk National University College of Medicine, Cheongju, Korea. wjkim@chungbuk.ac.kr
- KMID: 2363132
- DOI: http://doi.org/10.4111/icu.2016.57.2.106
Abstract
- PURPOSE
Topoisomerase-II alpha (TopoIIA ), a DNA gyrase isoform that plays an important role in the cell cycle, is present in normal tissues and various human cancers, and can show altered expression in both. The aim of the current study was to examine the value of urinary TopoIIA cell-free DNA as a noninvasive diagnosis of bladder cancer (BC).
MATERIALS AND METHODS
Two patient cohorts were examined. Cohort 1 (73 BC patients and seven controls) provided bladder tissue samples, whereas cohort 2 (83 BC patients, 54 nonmalignant hematuric patients, and 61 normal controls) provided urine samples. Real-time quantitative polymerase chain reaction was used to measure expression of TopoIIA mRNA in tissues and TopoIIA cell-free DNA in urine samples.
RESULTS
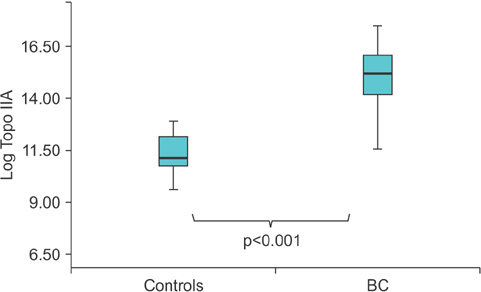
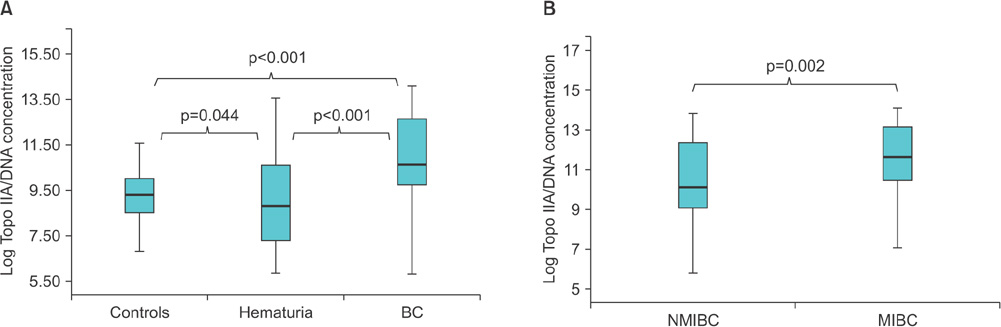
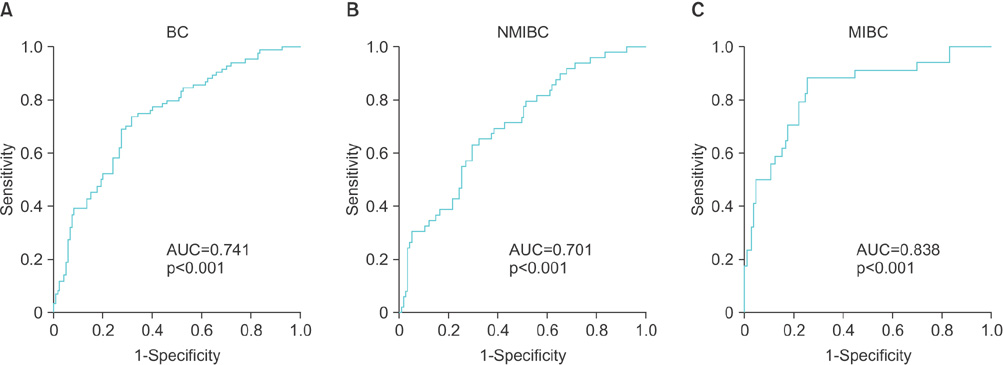
The results showed that expression of TopoIIA mRNA in BC tissues was significantly higher than that in noncancer control tissues (p<0.001). The expression of urinary TopoIIA cell-free DNA in BC patients was also significantly higher than that in noncancer patient controls and hematuria patients (p < 0.001 and p < 0.001, respectively). High expression of urinary TopoIIA cell-free DNA was also detected in muscle invasive bladder cancer (MIBC) when compared with nonmuscle invasive bladder cancer (NMIBC) (p=0.002). Receiver operating characteristics (ROC) curve analysis was performed to examine the sensitivity/specificity of urinary TopoIIA cell-free DNA for diagnosing BC, NMIBC, and MIBC. The areas under the ROC curve for BC, NMIBC, and MIBC were 0.741, 0.701, and 0.838, respectively.
CONCLUSIONS
In summary, the results of this study provide evidence that cell-free TopoIIA DNA may be a potential biomarker for BC.
MeSH Terms
-
Adult
Aged
Antigens, Neoplasm/genetics/*urine
Biomarkers, Tumor/genetics/*urine
Case-Control Studies
Cell-Free System
Cohort Studies
DNA Topoisomerases, Type II/genetics/*urine
DNA, Neoplasm/genetics
DNA-Binding Proteins/genetics/*urine
Female
Gene Expression
Hematuria/urine
Humans
Male
Middle Aged
Neoplasm Grading
Neoplasm Staging
RNA, Messenger/genetics
RNA, Neoplasm/genetics
Sensitivity and Specificity
Urinary Bladder Neoplasms/complications/*diagnosis/pathology
Antigens, Neoplasm
Biomarkers, Tumor
DNA Topoisomerases, Type II
DNA, Neoplasm
DNA-Binding Proteins
RNA, Messenger
RNA, Neoplasm
Figure
Reference
-
1. Jung KW, Won YJ, Oh CM, Kong HJ, Cho H, Lee DH, et al. Prediction of cancer incidence and mortality in Korea, 2015. Cancer Res Treat. 2015; 47:142–148.2. Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006; 49:466–465.3. Stenzl A, Cowan NC, De Santis M, Kuczyk MA, Merseburger AS, Ribal MJ, et al. Treatment of muscle-invasive and metastatic bladder cancer: update of the EAU guidelines. Eur Urol. 2011; 59:1009–1018.4. Messing EM, Vaillancourt A. Hematuria screening for bladder cancer. J Occup Med. 1990; 32:838–845.5. Marshall VF. Current clinical problems regarding bladder tumors. Cancer. 1956; 9:543–550.6. Messing EM, Young TB, Hunt VB, Roecker EB, Vaillancourt AM, Hisgen WJ, et al. Home screening for hematuria: results of a multiclinic study. J Urol. 1992; 148(2 Pt 1):289–292.7. Messing EM, Young TB, Hunt VB, Wehbie JM, Rust P. Urinary tract cancers found by homescreening with hematuria dipsticks in healthy men over 50 years of age. Cancer. 1989; 64:2361–2367.8. Strausfeld U, Richter A. Simultaneous purification of DNA topoisomerase I and II from eukaryotic cells. Prep Biochem. 1989; 19:37–48.9. Ohashi Y, Sasano H, Yamaki H, Shizawa S, Kikuchi A, Shineha R, et al. Topoisomerase II alpha expression in esophageal squamous cell carcinoma. Anticancer Res. 1999; 19(3A):1873–1880.10. Depowski PL, Rosenthal SI, Brien TP, Stylos S, Johnson RL, Ross JS. Topoisomerase IIalpha expression in breast cancer: correlation with outcome variables. Mod Pathol. 2000; 13:542–547.11. Costa MJ, Hansen CL, Holden JA, Guinee D Jr. Topoisomerase II alpha: prognostic predictor and cell cycle marker in surface epithelial neoplasms of the ovary and peritoneum. Int J Gynecol Pathol. 2000; 19:248–257.12. Dingemans AM, Witlox MA, Stallaert RA, van der Valk P, Postmus PE, Giaccone G. Expression of DNA topoisomerase IIalpha and topoisomerase IIbeta genes predicts survival and response to chemotherapy in patients with small cell lung cancer. Clin Cancer Res. 1999; 5:2048–2058.13. Nakopoulou L, Zervas A, Lazaris AC, Constantinides C, Stravodimos C, Davaris P, et al. Predictive value of topoisomerase II alpha immunostaining in urothelial bladder carcinoma. J Clin Pathol. 2001; 54:309–313.14. Kim EJ, Lee YS, Kim YJ, Kim MJ, Ha YS, Jeong P, et al. Clinical implications and prognostic values of topoisomerase-II alpha expression in primary non-muscle-invasive bladder cancer. Urology. 2010; 75:1516.e9–1516.e13.15. Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998; 22:1435–1448.16. Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Böhle A, Palou-Redorta J, et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Eur Urol. 2011; 59:997–1008.17. Schned AR, Andrew AS, Marsit CJ, Zens MS, Kelsey KT, Karagas MR. Survival following the diagnosis of noninvasive bladder cancer: WHO/International Society of Urological Pathology versus WHO classification systems. J Urol. 2007; 178(4 Pt 1):1196–1200.18. Yun SJ, Yan C, Jeong P, Kang HW, Kim YH, Kim EA, et al. Comparison of mRNA, protein, and urinary nucleic acid levels of S100A8 and S100A9 between prostate cancer and BPH. Ann Surg Oncol. 2015; 22:2439–2445.19. Lynch BJ, Guinee DG Jr, Holden JA. Human DNA topoisomerase II-alpha: a new marker of cell proliferation in invasive breast cancer. Hum Pathol. 1997; 28:1180–1188.20. Grossfeld GD, Litwin MS, Wolf JS Jr, Hricak H, Shuler CL, Agerter DC, et al. Evaluation of asymptomatic microscopic hematuria in adults: the American Urological Association best practice policy--part II: patient evaluation, cytology, voided markers, imaging, cystoscopy, nephrology evaluation, and follow-up. Urology. 2001; 57:604–610.21. Giannopoulos A, Manousakas T, Gounari A, Constantinides C, Choremi-Papadopoulou H, Dimopoulos C. Comparative evaluation of the diagnostic performance of the BTA stat test, NMP22 and urinary bladder cancer antigen for primary and recurrent bladder tumors. J Urol. 2001; 166:470–475.22. Simon MA, Lokeshwar VB, Soloway MS. Current bladder cancer tests: unnecessary or beneficial? Crit Rev Oncol Hematol. 2003; 47:91–107.23. van der Poel HG, Debruyne FM. Can biological markers replace cystoscopy? An update. Curr Opin Urol. 2001; 11:503–509.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative study on results between urine flow cytometric DNA analysis and urine cytology in transtional cell carcinoma of bladder
- Expression of DNA Topoisomerase II-alpha as a Proliferating Marker in Urothelial Carcinoma of Urinary Bladder based on World Health Organization/International Society of Urological Pathology Consensus Classification: A Correlation with Expression of Ki-67 and Apoptosis
- Liquid biopsy? A recent breakthrough in noninvasive bladder cancer surveillance
- The Expression of Topoisomerase II alpha in the Gastric Carcinoma
- Expression of DNA Topoisomerase II and P-Glycoprotein in Breast Cancer