Lab Anim Res.
2016 Dec;32(4):224-230. 10.5625/lar.2016.32.4.224.
Sirtuin-2 inhibition affects hippocampal functions and sodium butyrate ameliorates the reduction in novel object memory, cell proliferation, and neuroblast differentiation
- Affiliations
-
- 1Department of Anatomy and Cell Biology, College of Veterinary Medicine, and Research Institute for Veterinary Science, Seoul National University, Seoul, Korea. vetmed2@snu.ac.kr
- 2Department of Biochemistry and Molecular Biology, Research Institute of Oral Sciences, College of Dentistry, Gangneung-Wonju National University, Gangneung, Korea.
- 3Department of Anatomy, College of Veterinary Medicine, Kangwon National University, Chuncheon, Korea.
- 4Department of Veterinary Internal Medicine and Geriatrics, College of Veterinary Medicine, Kangwon National University, Chuncheon, Korea.
- 5Department of Neurobiology, School of Medicine, Kangwon National University, Chuncheon, Korea.
- KMID: 2362914
- DOI: http://doi.org/10.5625/lar.2016.32.4.224
Abstract
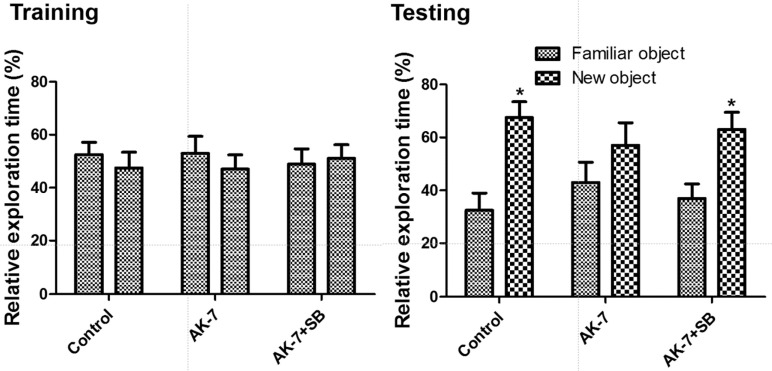
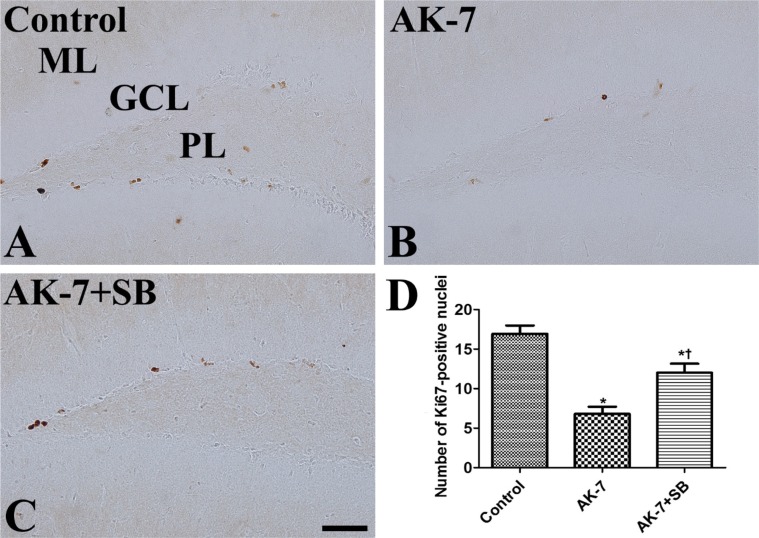
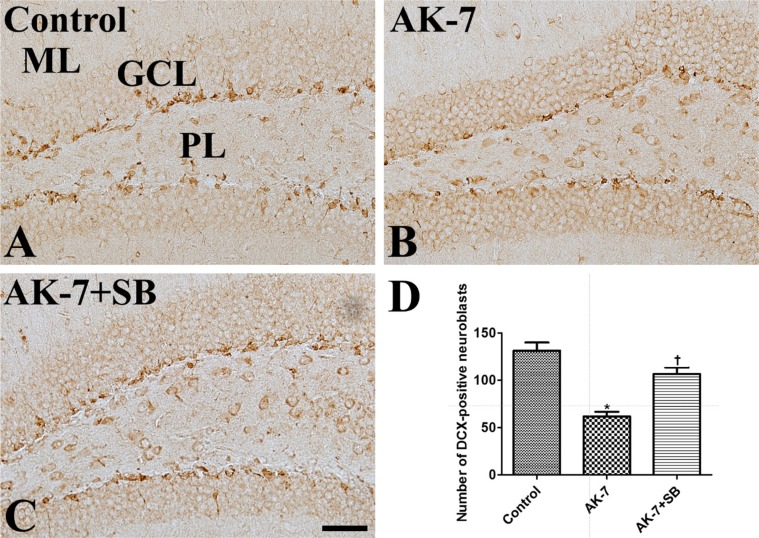
- We investigated the effects of the sirtuin-2 (SIRT2) inhibitor AK-7 on novel object memory, cell proliferation, and neuroblast differentiation in the dentate gyrus. In addition, we also observed the relationships with sodium butyrate, a histone deacetylase inhibitor, on the hippocampal functions. To investigate the effects of AK-7 on hippocampal functions, 10-week-old C57BL/6 mice were daily injected intraperitoneally with 20 mg/kg AK-7 alone or in combination with subcutaneous administration of 300 mg/kg sodium butyrate, a histone deacetylase inhibitor, for 21 days. A novel object recognition test was conducted on days 20 (training) and 21 (testing) of treatment. Thereafter, the animals were sacrificed for immunohistochemistry for Ki67 (cell proliferation) and doublecortin (DCX, neuroblast differentiation). AK-7 administration significantly reduced the time spent exploring new objects, while treatment in combination with sodium butyrate significantly alleviated this reduction. Additionally, AK-7 administration significantly reduced the number of Ki67-positive cells and DCX-immunoreactive neuroblasts in the dentate gyrus, while the treatment in combination with sodium butyrate ameliorated these changes. This result suggests that the reduction of SIRT2 may be closely related to age-related phenotypes including novel object memory, as well as cell proliferation and neuroblast differentiation in the dentate gyrus. In addition, sodium butyrate reverses SIRT2-related age phenotypes.
MeSH Terms
Figure
Reference
-
1. Crepaldi L, Riccio A. Chromatin learns to behave. Epigenetics. 2009; 4(1):23–26. PMID: 19197164.
Article2. Guan JS, Haggarty SJ, Giacometti E, Dannenberg JH, Joseph N, Gao J, Nieland TJ, Zhou Y, Wang X, Mazitschek R, Bradner JE, DePinho RA, Jaenisch R, Tsai LH. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009; 459(7243):55–60. PMID: 19424149.
Article3. Morrison BE, Majdzadeh N, D'Mello SR. Histone deacetylases: focus on the nervous system. Cell Mol Life Sci. 2007; 64(17):2258–2269. PMID: 17530170.
Article4. Dillin A, Kelly JW. Medicine. The yin-yang of sirtuins. Science. 2007; 317(5837):461–462. PMID: 17656709.5. Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009; 460(7255):587–591. PMID: 19641587.
Article6. Gan L, Mucke L. Paths of convergence: sirtuins in aging and neurodegeneration. Neuron. 2008; 58(1):10–14. PMID: 18400158.
Article7. de Oliveira RM, Pais TF, Outeiro TF. Sirtuins: common targets in aging and in neurodegeneration. Curr Drug Targets. 2010; 11(10):1270–1280. PMID: 20840069.8. Longo VD, Kennedy BK. Sirtuins in aging and age-related disease. Cell. 2006; 126(2):257–268. PMID: 16873059.
Article9. Liu L, Arun A, Ellis L, Peritore C, Donmez G. SIRT2 enhances 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced nigrostriatal damage via apoptotic pathway. Front Aging Neurosci. 2014; 6:184. PMID: 25157229.
Article10. Luthi-Carter R, Taylor DM, Pallos J, Lambert E, Amore A, Parker A, Moffitt H, Smith DL, Runne H, Gokce O, Kuhn A, Xiang Z, Maxwell MM, Reeves SA, Bates GP, Neri C, Thompson LM, Marsh JL, Kazantsev AG. SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proc Natl Acad Sci U S A. 2010; 107(17):7927–7932. PMID: 20378838.
Article11. Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, Kazantsev AG. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson's disease. Science. 2007; 317(5837):516–519. PMID: 17588900.12. Pandithage R, Lilischkis R, Harting K, Wolf A, Jedamzik B, Luscher-Firzlaff J, Vervoorts J, Lasonder E, Kremmer E, Knoll B, Luscher B. The regulation of SIRT2 function by cyclin-dependent kinases affects cell motility. J Cell Biol. 2008; 180(5):915–929. PMID: 18332217.
Article13. Yuan F, Xu ZM, Lu LY, Nie H, Ding J, Ying WH, Tian HL. SIRT2 inhibition exacerbates neuroinflammation and blood-brain barrier disruption in experimental traumatic brain injury by enhancing NF-êB p65 acetylation and activation. J Neurochem. 2016; 136(3):581–593. PMID: 26546505.
Article14. Chen X, Wales P, Quinti L, Zuo F, Moniot S, Herisson F, Rauf NA, Wang H, Silverman RB, Ayata C, Maxwell MM, Steegborn C, Schwarzschild MA, Outeiro TF, Kazantsev AG. The sirtuin-2 inhibitor AK7 is neuroprotective in models of Parkinson's disease but not amyotrophic lateral sclerosis and cerebral ischemia. PLoS One. 2015; 10(1):e0116919. PMID: 25608039.
Article15. Ramakrishnan G, Davaakhuu G, Kaplun L, Chung WC, Rana A, Atfi A, Miele L, Tzivion G. Sirt2 deacetylase is a novel AKT binding partner critical for AKT activation by insulin. J Biol Chem. 2014; 289(9):6054–6066. PMID: 24446434.
Article16. Derakhshan F, Toth C. Insulin and the brain. Curr Diabetes Rev. 2013; 9(2):102–116. PMID: 23231032.
Article17. Ziegler AN, Levison SW, Wood TL. Insulin and IGF receptor signalling in neural-stem-cell homeostasis. Nat Rev Endocrinol. 2015; 11(3):161–170. PMID: 25445849.
Article18. Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003; 133(7 Suppl):2485S–2493S. PMID: 12840228.
Article19. Valvassori SS, Varela RB, Arent CO, Dal-Pont GC, Bobsin TS, Budni J, Reus GZ, Quevedo J. Sodium butyrate functions as an antidepressant and improves cognition with enhanced neurotrophic expression in models of maternal deprivation and chronic mild stress. Curr Neurovasc Res. 2014; 11(4):359–366. PMID: 25233278.
Article20. Pandey K, Sharma KP, Sharma SK. Histone deacetylase inhibition facilitates massed pattern-induced synaptic plasticity and memory. Learn Mem. 2015; 22(10):514–518. PMID: 26373830.
Article21. Yoo DY, Kim DW, Kim MJ, Choi JH, Jung HY, Nam SM, Kim JW, Yoon YS, Choi SY, Hwang IK. Sodium butyrate, a histone deacetylase Inhibitor, ameliorates SIRT2-induced memory impairment, reduction of cell proliferation, and neuroblast differentiation in the dentate gyrus. Neurol Res. 2015; 37(1):69–76. PMID: 24963697.
Article22. Yoo DY, Kim W, Kim IH, Nam SM, Chung JY, Choi JH, Yoon YS, Won MH, Hwang IK. Combination effects of sodium butyrate and pyridoxine treatment on cell proliferation and neuroblast differentiation in the dentate gyrus of D-galactose-induced aging model mice. Neurochem Res. 2012; 37(1):223–231. PMID: 21984169.
Article23. Yoo DY, Kim W, Nam SM, Kim DW, Chung JY, Choi SY, Yoon YS, Won MH, Hwang IK. Synergistic effects of sodium butyrate, a histone deacetylase inhibitor, on increase of neurogenesis induced by pyridoxine and increase of neural proliferation in the mouse dentate gyrus. Neurochem Res. 2011; 36(10):1850–1857. PMID: 21597935.
Article24. Kim HJ, Leeds P, Chuang DM. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J Neurochem. 2009; 110(4):1226–1240. PMID: 19549282.
Article25. Yao X, Zhang JR, Huang HR, Dai LC, Liu QJ, Zhang M. Histone deacetylase inhibitor promotes differentiation of embryonic stem cells into neural cells in adherent monoculture. Chin Med J (Engl). 2010; 123(6):734–738. PMID: 20368096.26. Fessler EB, Chibane FL, Wang Z, Chuang DM. Potential roles of HDAC inhibitors in mitigating ischemia-induced brain damage and facilitating endogenous regeneration and recovery. Curr Pharm Des. 2013; 19(28):5105–5120. PMID: 23448466.
Article27. Clelland CD, Choi M, Romberg C, Clemenson GD Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009; 325(5937):210–213. PMID: 19590004.
Article28. Nakashiba T, Cushman JD, Pelkey KA, Renaudineau S, Buhl DL, McHugh TJ, Rodriguez Barrera V, Chittajallu R, Iwamoto KS, McBain CJ, Fanselow MS, Tonegawa S. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012; 149(1):188–201. PMID: 22365813.
Article29. Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011; 472(7344):466–470. PMID: 21460835.
Article30. Drapeau E, Nora Abrous D. Stem cell review series: role of neurogenesis in age-related memory disorders. Aging Cell. 2008; 7(4):569–589. PMID: 18221417.
Article31. Ngwenya LB, Heyworth NC, Shwe Y, Moore TL, Rosene DL. Age-related changes in dentate gyrus cell numbers, neurogenesis, and associations with cognitive impairments in the rhesus monkey. Front Syst Neurosci. 2015; 9:102. PMID: 26236203.
Article32. Braidy N, Poljak A, Grant R, Jayasena T, Mansour H, Chan-Ling T, Smythe G, Sachdev P, Guillemin GJ. Differential expression of sirtuins in the aging rat brain. Front Cell Neurosci. 2015; 9:167. PMID: 26005404.
Article33. Kireev RA, Vara E, Tresguerres JA. Growth hormone and melatonin prevent age-related alteration in apoptosis processes in the dentate gyrus of male rats. Biogerontology. 2013; 14(4):431–442. PMID: 23852044.
Article34. Taylor DM, Balabadra U, Xiang Z, Woodman B, Meade S, Amore A, Maxwell MM, Reeves S, Bates GP, Luthi-Carter R, Lowden PA, Kazantsev AG. A brain-permeable small molecule reduces neuronal cholesterol by inhibiting activity of sirtuin 2 deacetylase. ACS Chem Biol. 2011; 6(6):540–546. PMID: 21370928.
Article35. Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003; 467(1):1–10. PMID: 14574675.
Article36. Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005; 21(1):1–14. PMID: 15654838.
Article37. Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press;1997.38. Liu R, Dang W, Du Y, Zhou Q, Jiao K, Liu Z. SIRT2 is involved in the modulation of depressive behaviors. Sci Rep. 2015; 5:8415. PMID: 25672834.
Article39. Erburu M, Munoz-Cobo I, Dominguez-Andres J, Beltran E, Suzuki T, Mai A, Valente S, Puerta E, Tordera RM. Chronic stress and antidepressant induced changes in Hdac5 and Sirt2 affect synaptic plasticity. Eur Neuropsychopharmacol. 2015; 25(11):2036–2048. PMID: 26433268.
Article40. Pfister JA, Ma C, Morrison BE, D'Mello SR. Opposing effects of sirtuins on neuronal survival: SIRT1-mediated neuroprotection is independent of its deacetylase activity. PLoS One. 2008; 3(12):e4090. PMID: 19116652.
Article41. Chopra V, Quinti L, Kim J, Vollor L, Narayanan KL, Edgerly C, Cipicchio PM, Lauver MA, Choi SH, Silverman RB, Ferrante RJ, Hersch S, Kazantsev AG. The sirtuin 2 inhibitor AK-7 is neuroprotective in Huntington's disease mouse models. Cell Rep. 2012; 2(6):1492–1497. PMID: 23200855.
Article42. Pais TF, Szegõ ÉM, Marques O, Miller-Fleming L, Antas P, Guerreiro P, de Oliveira RM, Kasapoglu B, Outeiro TF. The NAD-dependent deacetylase sirtuin 2 is a suppressor of microglial activation and brain inflammation. EMBO J. 2013; 32(19):2603–2616. PMID: 24013120.
Article43. Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD. Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem. 2004; 279(39):40545–40559. PMID: 15273246.
Article44. Vecsey CG, Hawk JD, Lattal KM, Stein JM, Fabian SA, Attner MA, Cabrera SM, McDonough CB, Brindle PK, Abel T, Wood MA. Histone deacetylase inhibitors enhance memory and synaptic plasticity via CREB:CBP-dependent transcriptional activation. J Neurosci. 2007; 27(23):6128–6140. PMID: 17553985.45. Gundersen BB, Blendy JA. Effects of the histone deacetylase inhibitor sodium butyrate in models of depression and anxiety. Neuropharmacology. 2009; 57(1):67–74. PMID: 19393671.
Article46. Takuma K, Hara Y, Kataoka S, Kawanai T, Maeda Y, Watanabe R, Takano E, Hayata-Takano A, Hashimoto H, Ago Y, Matsuda T. Chronic treatment with valproic acid or sodium butyrate attenuates novel object recognition deficits and hippocampal dendritic spine loss in a mouse model of autism. Pharmacol Biochem Behav. 2014; 126:43–49. PMID: 25240644.
Article47. Valvassori SS, Calixto KV, Budni J, Resende WR, Varela RB, de Freitas KV, Goncalves CL, Streck EL, Quevedo J. Sodium butyrate reverses the inhibition of Krebs cycle enzymes induced by amphetamine in the rat brain. J Neural Transm (Vienna). 2013; 120(12):1737–1742. PMID: 23851624.
Article48. Rana P, Gupta M, Khan AR, Hemanth Kumar BS, Roy R, Khushu S. NMR based metabolomics reveals acute hippocampal metabolic fluctuations during cranial irradiation in murine model. Neurochem Int. 2014; 74:1–7. PMID: 24787771.
Article49. Achanta P, Fuss M, Martinez JL Jr. Ionizing radiation impairs the formation of trace fear memories and reduces hippocampal neurogenesis. Behav Neurosci. 2009; 123(5):1036–1045. PMID: 19824769.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Vanillin and 4-hydroxybenzyl alcohol attenuate cognitive impairment and the reduction of cell proliferation and neuroblast differentiation in the dentate gyrus in a mouse model of scopolamine-induced amnesia
- In vitro Induction of Cellular Differentiation of Human Fetal Liver Cell Lines with Sodium Butyrate
- Bacopa monnieri extract improves novel object recognition, cell proliferation, neuroblast differentiation, brain-derived neurotrophic factor, and phosphorylation of cAMP response element-binding protein in the dentate gyrus
- Comparison of pharmacological and genetic inhibition of cyclooxygenase-2: effects on adult neurogenesis in the hippocampal dentate gyrus
- Heat shock protein 70 increases cell proliferation, neuroblast differentiation, and the phosphorylation of CREB in the hippocampus