Anat Cell Biol.
2017 Jun;50(2):143-151. 10.5115/acb.2017.50.2.143.
Vanillin and 4-hydroxybenzyl alcohol attenuate cognitive impairment and the reduction of cell proliferation and neuroblast differentiation in the dentate gyrus in a mouse model of scopolamine-induced amnesia
- Affiliations
-
- 1Department of Surgery, Kangwon National University School of Medicine, Chuncheon, Korea.
- 2Department of Biomedical Science, Research Institute of Bioscience and Biotechnology, Hallym University, Chuncheon, Korea. jh-park@hallym.ac.kr
- KMID: 2451240
- DOI: http://doi.org/10.5115/acb.2017.50.2.143
Abstract
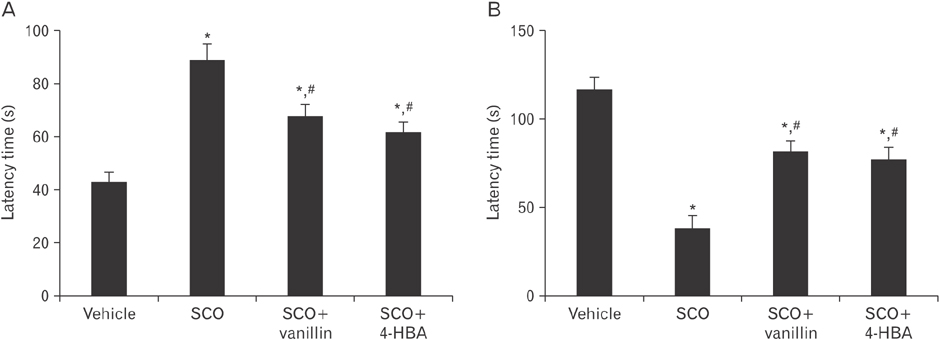
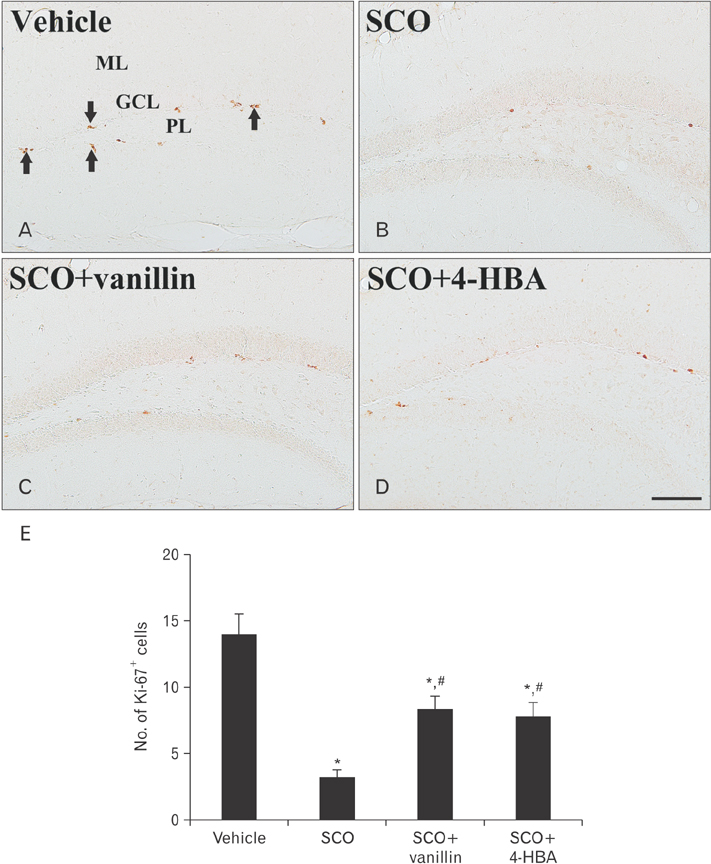
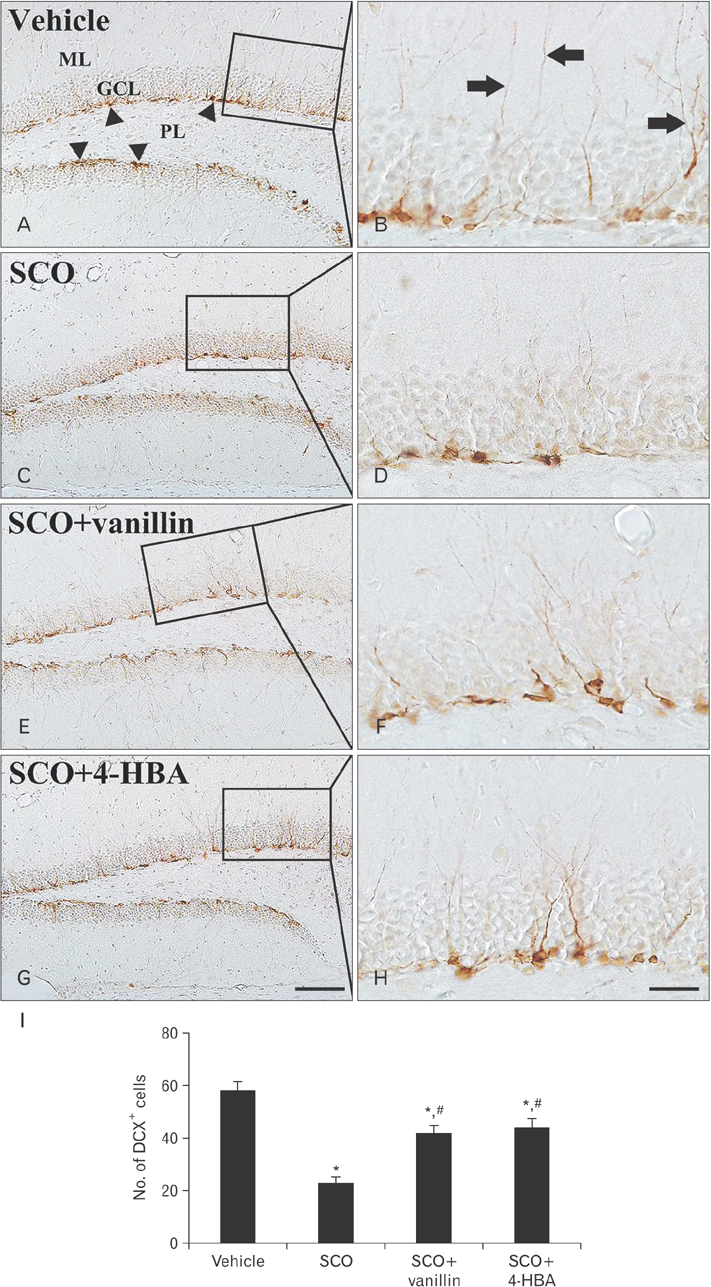
- 4-Hydroxy-3-methoxybenzaldehyde (vanillin) and 4-hydroxybenzyl alcohol (4-HBA) are natural phenolic compounds, which present in many plants and have diverse biological properties. In this study, we examined effects of vanillin and 4-HBA on learning and memory function, cell proliferation, and neuroblast differentiation in the hippocampal dentate gyrus in a mouse model of scopolamine-induced amnesia. Scopolamine (SCO; 1 mg/kg/day, intraperitoneally), vanillin, and 4-HBA (40 mg/kg/day, orally) were administered for 28 days. Treatment with scopolamine alone impaired learning and memory function in the Morris water maze and passive avoidance tests, in addition, the treatment significantly reduced cell proliferation and neuroblast differentiation in the dentate gyrus, which were examined by immunohistochemistry for Ki-67 (a classic marker for cell proliferation) and doublecortin (a marker for neuroblasts). However, treatment with vanillin or 4-HBA significantly attenuated SCO-induced learning and memory impairment as well as the reduction of cell proliferation and neuroblast differentiation in the dentate gyrus. These results indicate that vanillin and 4-HBA may be helpful in improving cognitive function and in increasing endogenous neuronal proliferation in the brain.
MeSH Terms
Figure
Cited by 2 articles
-
Morphine-alcohol treatment impairs cognitive functions and increases neuro-inflammatory responses in the medial prefrontal cortex of juvenile male rats
Adekomi Damilare Adedayo, Adegoke Adebiyi Aderinola, Tijani Ahmad Adekilekun, Olaniyan Olayinka Olaolu, Alabi Mutiyat Olanike, Ijomone Kafilat Olayemi
Anat Cell Biol. 2018;51(1):41-51. doi: 10.5115/acb.2018.51.1.41.Large-scale functional brain networks for consciousness
Myoung-Eun Han, Si-Young Park, Sae-Ock Oh
Anat Cell Biol. 2021;54(2):152-164. doi: 10.5115/acb.20.305.
Reference
-
1. Blokland A. Acetylcholine: a neurotransmitter for learning and memory? Brain Res Brain Res Rev. 1995; 21:285–300.2. Blusztajn JK, Wurtman RJ. Choline and cholinergic neurons. Science. 1983; 221:614–620.3. Bartus RT, Dean RL 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982; 217:408–414.4. Mohapel P, Leanza G, Kokaia M, Lindvall O. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiol Aging. 2005; 26:939–946.5. Falsafi SK, Deli A, Höger H, Pollak A, Lubec G. Scopolamine administration modulates muscarinic, nicotinic and NMDA receptor systems. PLoS One. 2012; 7:e32082.6. Abe E. Reversal effect of DM-9384 on scopolamine-induced acetylcholine depletion in certain regions of the mouse brain. Psychopharmacology (Berl). 1991; 105:310–316.7. Lee S, Kim J, Seo SG, Choi BR, Han JS, Lee KW, Kim J. Sulforaphane alleviates scopolamine-induced memory impairment in mice. Pharmacol Res. 2014; 85:23–32.8. Cunha GM, Canas PM, Melo CS, Hockemeyer J, Müller CE, Oliveira CR, Cunha RA. Adenosine A2A receptor blockade prevents memory dysfunction caused by beta-amyloid peptides but not by scopolamine or MK-801. Exp Neurol. 2008; 210:776–781.9. Manral A, Meena P, Saini V, Siraj F, Shalini S, Tiwari M. DADS analogues ameliorated the cognitive impairments of Alzheimer-like rat model induced by scopolamine. Neurotox Res. 2016; 30:407–426.10. Cao Y, Zhang X, Fang Y, Ye J. Determination of the active ingredients in Gastrodia rhizoma by capillary electrophoresis with electrochemical detection. Analyst. 2001; 126:1524–1528.11. Yang XD, Zhu J, Yang R, Liu JP, Li L, Zhang HB. Phenolic constituents from the rhizomes of Gastrodia elata. Nat Prod Res. 2007; 21:180–186.12. Jung TY, Suh SI, Lee H, Kim IS, Kim HJ, Yoo HS, Lee SR. Protective effects of several components of Gastrodia elata on lipid peroxidation in gerbil brain homogenates. Phytother Res. 2007; 21:960–964.13. Kim NH, Xin MJ, Cha JY, Ji SJ, Kwon SU, Jee HK, Park MR, Park YS, Kim CT, Kim DK, Lee YM. Antitumor and immunomodulatory effect of Gastrodia elata on colon cancer in vitro and In vivo. Am J Chin Med. 2017; 45:319–335.14. Lee JY, Jang YW, Kang HS, Moon H, Sim SS, Kim CJ. Anti-inflammatory action of phenolic compounds from Gastrodia elata root. Arch Pharm Res. 2006; 29:849–858.15. Lirdprapamongkol K, Sakurai H, Kawasaki N, Choo MK, Saitoh Y, Aozuka Y, Singhirunnusorn P, Ruchirawat S, Svasti J, Saiki I. Vanillin suppresses in vitro invasion and in vivo metastasis of mouse breast cancer cells. Eur J Pharm Sci. 2005; 25:57–65.16. Lee YS, Ha JH, Yong CS, Lee DU, Huh K, Kang YS, Lee SH, Jung MW, Kim JA. Inhibitory effects of constituents of Gastrodia elata Bl. on glutamate-induced apoptosis in IMR-32 human neuroblastoma cells. Arch Pharm Res. 1999; 22:404–409.17. Kim HJ, Hwang IK, Won MH. Vanillin, 4-hydroxybenzyl aldehyde and 4-hydroxybenzyl alcohol prevent hippocampal CA1 cell death following global ischemia. Brain Res. 2007; 1181:130–141.18. Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010; 11:339–350.19. Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: a possible role in learning. Trends Cogn Sci. 1999; 3:186–192.20. Cho JH, Park JH, Ahn JH, Lee JC, Hwang IK, Park SM, Ahn JY, Kim DW, Cho JH, Kim JD, Kim YM, Won MH, Kang IJ. Vanillin and 4-hydroxybenzyl alcohol promotes cell proliferation and neuroblast differentiation in the dentate gyrus of mice via the increase of brain-derived neurotrophic factor and tropomyosin-related kinase B. Mol Med Rep. 2016; 13:2949–2956.21. Institute of Laboratory Animal Research. Committee for the Update of the Guide for the Care and Use of Laboratory Animals. National Research Council. Guide for the care and use of laboratory animals. 8th ed. Washington, DC: National Academies Press;2011. p. 220.22. Park JH, Choi HY, Cho JH, Kim IH, Lee TK, Lee JC, Won MH, Chen BH, Shin BN, Ahn JH, Tae HJ, Choi JH, Chung JY, Lee CH, Kang IJ, Kim JD. Effects of chronic scopolamine treatment on cognitive impairments and myelin basic protein expression in the mouse hippocampus. J Mol Neurosci. 2016; 59:579–589.23. Lee JC, Park JH, Ahn JH, Kim IH, Cho JH, Choi JH, Yoo KY, Lee CH, Hwang IK, Cho JH, Kwon YG, Kim YM, Kang IJ, Won MH. New GABAergic neurogenesis in the hippocampal CA1 region of a gerbil model of long-term survival after transient cerebral ischemic injury. Brain Pathol. 2016; 26:581–592.24. Yan BC, Park JH, Chen BH, Cho JH, Kim IH, Ahn JH, Lee JC, Hwang IK, Cho JH, Lee YL, Kang IJ, Won MH. Long-term administration of scopolamine interferes with nerve cell proliferation, differentiation and migration in adult mouse hippocampal dentate gyrus, but it does not induce cell death. Neural Regen Res. 2014; 9:1731–1739.25. Yan BC, Kim IH, Park JH, Ahn JH, Cho JH, Chen BH, Lee JC, Choi JH, Yoo KY, Lee CH, Cho JH, Kim JD, Won MH. Systemic administration of low dosage of tetanus toxin decreases cell proliferation and neuroblast differentiation in the mouse hippocampal dentate gyrus. Lab Anim Res. 2013; 29:148–155.26. Paxinos G, Franklin KB. The mouse brain in stereotaxic coordinates. San Diego, CA: Academic Press;1997.27. Vasileva LV, Getova DP, Doncheva ND, Marchev AS, Georgiev MI. Beneficial effect of commercial Rhodiola extract in rats with scopolamine-induced memory impairment on active avoidance. J Ethnopharmacol. 2016; 193:586–591.28. Gupta S, Sharma B. Pharmacological benefits of agomelatine and vanillin in experimental model of Huntington's disease. Pharmacol Biochem Behav. 2014; 122:122–135.29. Gupta S, Sharma B, Singh P, Sharma BM. Modulation of transient receptor potential vanilloid subtype 1 (TRPV1) and norepinephrine transporters (NET) protect against oxidative stress, cellular injury, and vascular dementia. Curr Neurovasc Res. 2014; 11:94–106.30. Hsieh MT, Wu CR, Chen CF. Gastrodin and p-hydroxybenzyl alcohol facilitate memory consolidation and retrieval, but not acquisition, on the passive avoidance task in rats. J Ethnopharmacol. 1997; 56:45–54.31. Goel V, Makale M, Grafman J. The hippocampal system mediates logical reasoning about familiar spatial environments. J Cogn Neurosci. 2004; 16:654–664.32. Jarrard LE. On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol. 1993; 60:9–26.33. Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001; 410:372–376.34. Yoo DY, Kim W, Yoo KY, Lee CH, Choi JH, Kang IJ, Yoon YS, Kim DW, Won MH, Hwang IK. Effects of Nelumbo nucifera rhizome extract on cell proliferation and neuroblast differentiation in the hippocampal dentate gyrus in a scopolamine-induced amnesia animal model. Phytother Res. 2011; 25:809–815.35. Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005; 21:1–14.36. Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods. 2002; 115:97–105.37. Plumpe T, Ehninger D, Steiner B, Klempin F, Jessberger S, Brandt M, Römer B, Rodriguez GR, Kronenberg G, Kempermann G. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci. 2006; 7:77.38. Ramirez-Rodriguez G, Ortíz-López L, Domínguez-Alonso A, Benítez-King GA, Kempermann G. Chronic treatment with melatonin stimulates dendrite maturation and complexity in adult hippocampal neurogenesis of mice. J Pineal Res. 2011; 50:29–37.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bacopa monnieri extract improves novel object recognition, cell proliferation, neuroblast differentiation, brain-derived neurotrophic factor, and phosphorylation of cAMP response element-binding protein in the dentate gyrus
- Temporal Change of Calbindin-D28k Immunoreactivity in the Dentate Gyrus of Voluntary Running Mouse
- A Novel Histone Deacetylase 6 Inhibitor, 4-FHA, Improves Scopolamine-Induced Cognitive and Memory Impairment in Mice
- The high dosage of earthworm (Eisenia andrei) extract decreases cell proliferation and neuroblast differentiation in the mouse hippocampal dentate gyrus
- Comparison of pharmacological and genetic inhibition of cyclooxygenase-2: effects on adult neurogenesis in the hippocampal dentate gyrus