Lab Anim Res.
2018 Dec;34(4):239-247. 10.5625/lar.2018.34.4.239.
Bacopa monnieri extract improves novel object recognition, cell proliferation, neuroblast differentiation, brain-derived neurotrophic factor, and phosphorylation of cAMP response element-binding protein in the dentate gyrus
- Affiliations
-
- 1Department of Biochemistry and Molecular Biology, Research Institute of Oral Sciences, College of Dentistry, Gangneung-Wonju National University, Gangneung, Korea. kimdw@gwnu.ac.kr
- 2Department of Anatomy and Cell Biology, College of Veterinary Medicine, and Research Institute for Veterinary Science, Seoul National University, Seoul, Korea.
- 3Department of Anatomy, College of Medicine, Soonchunhyang University, Cheonan, Korea.
- KMID: 2459301
- DOI: http://doi.org/10.5625/lar.2018.34.4.239
Abstract
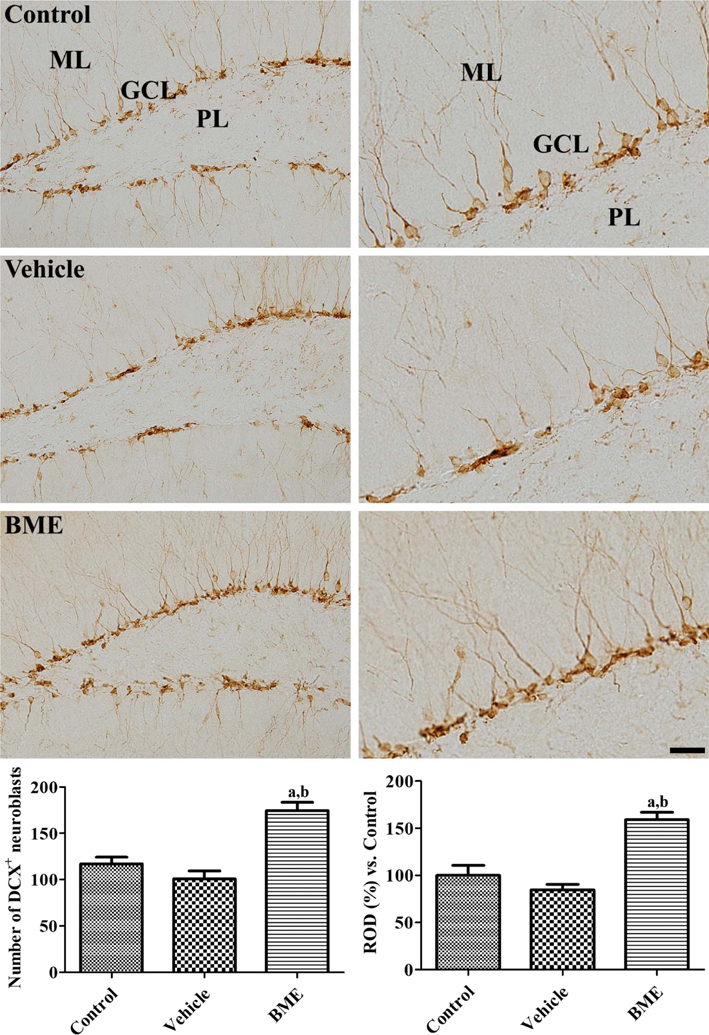
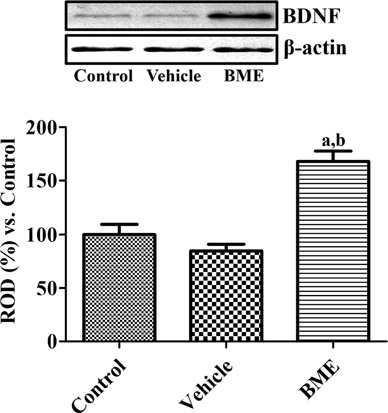
- Bacopa monnieri is a medicinal plant with a long history of use in Ayurveda, especially in the treatment of poor memory and cognitive deficits. In the present study, we hypothesized that Bacopa monnieri extract (BME) can improve memory via increased cell proliferation and neuroblast differentiation in the dentate gyrus. BME was administered to 7-week-old mice once a day for 4 weeks and a novel object recognition memory test was performed. Thereafter, the mice were euthanized followed by immunohistochemistry analysis for Ki67, doublecortin (DCX), and phosphorylated cAMP response element-binding protein (CREB), and western blot analysis of brain-derived neurotrophic factor (BDNF). BME-treated mice showed moderate increases in the exploration of new objects when compared with that of familiar objects, leading to a significant higher discrimination index compared with vehicle-treated mice. Ki67 and DCX immunohistochemistry showed a facilitation of cell proliferation and neuroblast differentiation following the administration of BME in the dentate gyrus. In addition, administration of BME significantly elevated the BDNF protein expression in the hippocampal dentate gyrus, and increased CREB phosphorylation in the dentate gyrus. These data suggest that BME improves novel object recognition by increasing the cell proliferation and neuroblast differentiation in the dentate gyrus, and this may be closely related to elevated levels of BDNF and CREB phosphorylation in the dentate gyrus.
Keyword
MeSH Terms
-
Animals
Bacopa*
Blotting, Western
Brain-Derived Neurotrophic Factor*
Cell Proliferation*
Cognition Disorders
Cyclic AMP Response Element-Binding Protein*
Dentate Gyrus*
Discrimination (Psychology)
Immunohistochemistry
Memory
Mice
Neurogenesis
Phosphorylation*
Plants, Medicinal
Brain-Derived Neurotrophic Factor
Cyclic AMP Response Element-Binding Protein
Figure
Reference
-
1. Khalaf-Nazzal R, Francis F. Hippocampal development-old and new findings. Neuroscience. 2013; 248:225–242.2. Atri A, Sherman S, Norman KA, Kirchhoff BA, Nicolas MM, Greicius MD, Cramer SC, Breiter HC, Hasselmo ME, Stern CE. Blockade of central cholinergic receptors impairs new learning and increases proactive interference in a word paired-associate memory task. Behav Neurosci. 2004; 118(1):223–236.
Article3. Hasselmo ME, McGaughy J. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Prog Brain Res. 2004; 145:207–231.
Article4. Buccafusco JJ, Letchworth SR, Bencherif M, Lippiello PM. Long-lasting cognitive improvement with nicotinic receptor agonists: mechanisms of pharmacokinetic-pharmacodynamic discordance. Trends Pharmacol Sci. 2005; 26(7):352–360.
Article5. Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl). 2006; 184(3-4):523–539.
Article6. Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998; 4(11):1313–1317.
Article7. Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999; 2(3):260–265.
Article8. Alam MJ, Kitamura T, Saitoh Y, Ohkawa N, Kondo T, Inokuchi K. Adult Neurogenesis Conserves Hippocampal Memory Capacity. J Neurosci. 2018; 38(31):6854–6863.
Article9. Aguiar S, Borowski T. Neuropharmacological review of the nootropic herb Bacopa monnieri. Rejuvenation Res. 2013; 16(4):313–326.10. Rastogi S, Pal R, Kulshreshtha DK. Bacoside A3--a triterpenoid saponin from Bacopa monniera. Phytochemistry. 1994; 36(1):133–137.11. Garai S, Mahato SB, Ohtani K, Yamasaki K. Bacopasaponin D--a pseudojujubogenin glycoside from Bacopa monniera. Phytochemistry. 1996; 43(2):447–449.12. Saraf MK, Anand A, Prabhakar S. Scopolamine induced amnesia is reversed by Bacopa monniera through participation of kinase-CREB pathway. Neurochem Res. 2010; 35(2):279–287.13. Liu X, Yue R, Zhang J, Shan L, Wang R, Zhang W. Neuroprotective effects of bacopaside I in ischemic brain injury. Restor Neurol Neurosci. 2013; 31(2):109–123.
Article14. Pase MP, Kean J, Sarris J, Neale C, Scholey AB, Stough C. The cognitive-enhancing effects of Bacopa monnieri: a systematic review of randomized, controlled human clinical trials. J Altern Complement Med. 2012; 18(7):647–652.
Article15. Kumar S, Mondal AC. Neuroprotective, Neurotrophic and Anti-oxidative Role of Bacopa monnieri on CUS Induced Model of Depression in Rat. Neurochem Res. 2016; 41(11):3083–3094.
Article16. Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010; 8(6):e1000412.
Article17. Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003; 467(1):1–10.
Article18. Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005; 21(1):1–14.
Article19. Rani A, Prasad S. A Special Extract of Bacopa monnieri (CDRI-08)-Restored Memory in CoCl2-Hypoxia Mimetic Mice Is Associated with Upregulation of Fmr-1 Gene Expression in Hippocampus. Evid Based Complement Alternat Med. 2015; 2015:347978.20. Jung HY, Kim DW, Nam SM, Kim JW, Chung JY, Won MH, Seong JK, Yoon YS, Yoo DY, Hwang IK. Pyridoxine improves hippocampal cognitive function via increases of serotonin turnover and tyrosine hydroxylase, and its association with CB1 cannabinoid receptor-interacting protein and the CB1 cannabinoid receptor pathway. Biochim Biophys Acta Gen Subj. 2017; 1861(12):3142–3153.
Article21. Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego: Academic Press;2001.22. Sairam K, Rao CV, Babu MD, Goel RK. Prophylactic and curative effects of Bacopa monniera in gastric ulcer models. Phytomedicine. 2001; 8(6):423–430.23. Kumar V. Potential medicinal plants for CNS disorders: an overview. Phytother Res. 2006; 20(12):1023–1035.
Article24. Pandey SP, Singh HK, Prasad S. Alterations in Hippocampal Oxidative Stress, Expression of AMPA Receptor GluR2 Subunit and Associated Spatial Memory Loss by Bacopa monnieri Extract (CDRI-08) in Streptozotocin-Induced Diabetes Mellitus Type 2 Mice. PLoS One. 2015; 10(7):e0131862.
Article25. Chaudhari KS, Tiwari NR, Tiwari RR, Sharma RS. Neurocognitive Effect of Nootropic Drug Brahmi (Bacopa monnieri) in Alzheimer's Disease. Ann Neurosci. 2017; 24(2):111–122.26. Saraf MK, Prabhakar S, Khanduja KL, Anand A. Bacopa monniera Attenuates Scopolamine-Induced Impairment of Spatial Memory in Mice. Evid Based Complement Alternat Med. 2011; 2011:236186.27. Stough C, Lloyd J, Clarke J, Downey LA, Hutchison CW, Rodgers T, Nathan PJ. The chronic effects of an extract of Bacopa monniera (Brahmi) on cognitive function in healthy human subjects. Psychopharmacology (Berl). 2001; 156(4):481–484.28. Peth-Nui T, Wattanathorn J, Muchimapura S, Tong-Un T, Piyavhatkul N, Rangseekajee P, Ingkaninan K, Vittaya-Areekul S. Effects of 12-Week Bacopa monnieri Consumption on Attention, Cognitive Processing, Working Memory, and Functions of Both Cholinergic and Monoaminergic Systems in Healthy Elderly Volunteers. Evid Based Complement Alternat Med. 2012; 2012:606424.29. Riedel G, Micheau J, Lam AG, Roloff EL, Martin SJ, Bridge H, de Hoz L, Poeschel B, McCulloch J, Morris RG. Reversible neural inactivation reveals hippocampal participation in several memory processes. Nat Neurosci. 1999; 2(10):898–905.
Article30. Promsuban C, Limsuvan S, Akarasereenont P, Tilokskulchai K, Tapechum S, Pakaprot N. Bacopa monnieri extract enhances learning-dependent hippocampal long-term synaptic potentiation. Neuroreport. 2017; 28(16):1031–1035.31. Lindholm JS, Castrén E. Mice with altered BDNF signaling as models for mood disorders and antidepressant effects. Front Behav Neurosci. 2014; 8:143.
Article32. Suzuki A, Fukushima H, Mukawa T, Toyoda H, Wu LJ, Zhao MG, Xu H, Shang Y, Endoh K, Iwamoto T, Mamiya N, Okano E, Hasegawa S, Mercaldo V, Zhang Y, Maeda R, Ohta M, Josselyn SA, Zhuo M, Kida S. Upregulation of CREB-mediated transcription enhances both short- and long-term memory. J Neurosci. 2011; 31(24):8786–8802.
Article33. Kida S, Serita T. Functional roles of CREB as a positive regulator in the formation and enhancement of memory. Brain Res Bull. 2014; 105:17–24.
Article34. Konar A, Gautam A, Thakur MK. Bacopa monniera (CDRI-08) Upregulates the Expression of Neuronal and Glial Plasticity Markers in the Brain of Scopolamine Induced Amnesic Mice. Evid Based Complement Alternat Med. 2015; 2015:837012.35. Ortega-Martínez S. A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Front Mol Neurosci. 2015; 8:46.
Article36. Hazra S, Kumar S, Saha GK, Mondal AC. Reversion of BDNF, Akt and CREB in Hippocampus of Chronic Unpredictable Stress Induced Rats: Effects of Phytochemical, Bacopa Monnieri. Psychiatry Investig. 2017; 14(1):74–80.37. Preethi J, Singh HK, Venkataraman JS, Rajan KE. Standardised extract of Bacopa monniera (CDRI-08) improves contextual fear memory by differentially regulating the activity of histone acetylation and protein phosphatases (PP1α, PP2A) in hippocampus. Cell Mol Neurobiol. 2014; 34(4):577–589.
Article38. Preethi J, Singh HK, Rajan KE. Possible Involvement of Standardized Bacopa monniera Extract (CDRI-08) in Epigenetic Regulation of reelin and Brain-Derived Neurotrophic Factor to Enhance Memory. Front Pharmacol. 2016; 7:166.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Heat shock protein 70 increases cell proliferation, neuroblast differentiation, and the phosphorylation of CREB in the hippocampus
- The high dosage of earthworm (Eisenia andrei) extract decreases cell proliferation and neuroblast differentiation in the mouse hippocampal dentate gyrus
- Administration of Phytoceramide Enhances Memory and Upregulates the Expression of pCREB and BDNF in Hippocampus of Mice
- Reversion of BDNF, Akt and CREB in Hippocampus of Chronic Unpredictable Stress Induced Rats: Effects of Phytochemical, Bacopa Monnieri
- Vanillin and 4-hydroxybenzyl alcohol attenuate cognitive impairment and the reduction of cell proliferation and neuroblast differentiation in the dentate gyrus in a mouse model of scopolamine-induced amnesia