J Vet Sci.
2015 Sep;16(3):245-251. 10.4142/jvs.2015.16.3.245.
Comparison of pharmacological and genetic inhibition of cyclooxygenase-2: effects on adult neurogenesis in the hippocampal dentate gyrus
- Affiliations
-
- 1Department of Anatomy and Cell Biology, College of Veterinary Medicine, Seoul National University, Seoul 151-742, Korea. ysyoon@snu.ac.kr
- 2BK21 PLUS Program for Creative Veterinary Science Research, and Research Institute for Veterinary Science, Seoul National University, Seoul 151-742, Korea.
- 3Department of Anatomy, College of Veterinary Medicine, Kangwon National University, Chuncheon 200-701, Korea.
- 4Department of Neurobiology, School of Medicine, Kangwon National University, Chuncheon 200-701, Korea.
- 5Korea Mouse Phenotyping Center, Seoul National University, Seoul 151-742, Korea.
- KMID: 2344296
- DOI: http://doi.org/10.4142/jvs.2015.16.3.245
Abstract
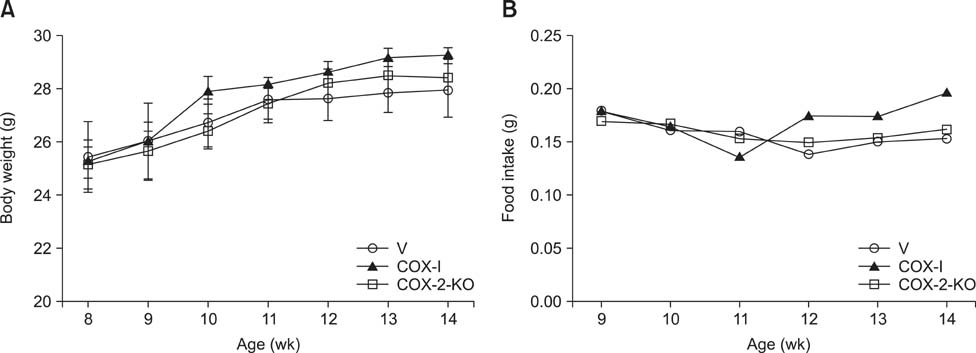
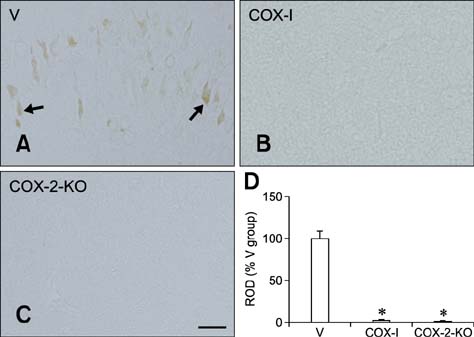
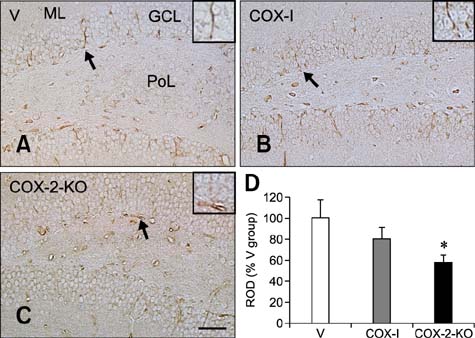
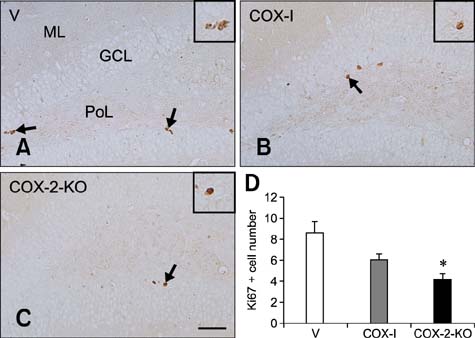
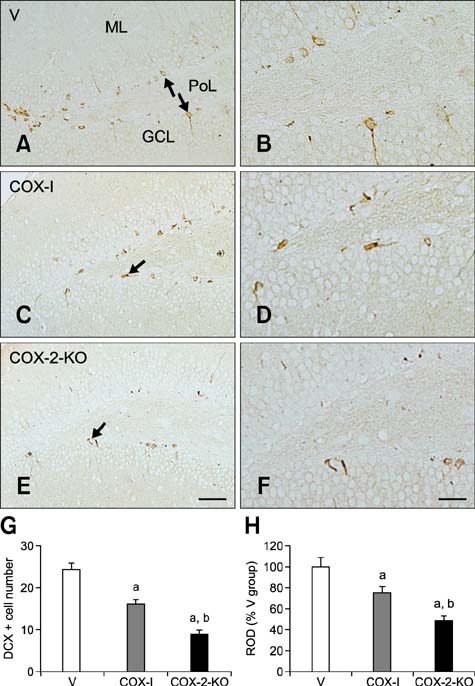
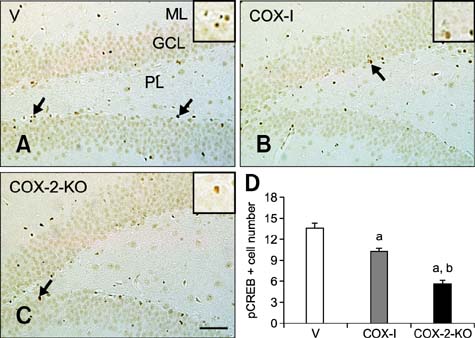
- Inducible cyclooxygenase-2 (COX-2) has received much attention because of its role in neuro-inflammation and synaptic plasticity. Even though COX-2 levels are high in healthy animals, the function of this factor in adult neurogenesis has not been clearly demonstrated. Therefore, we performed the present study to compare the effects of pharmacological and genetic inhibition of COX-2 on adult hippocampal neurogenesis. Physiological saline or the same volume containing celecoxib was administered perorally every day for 5 weeks using a feeding needle. Compared to the control, pharmacological and genetic inhibition of COX-2 reduced the appearance of nestin-immunoreactive neural stem cells, Ki67-positive nuclei, and doublecortin-immunoreactive neuroblasts in the dentate gyrus. In addition, a decrease in phosphorylated cAMP response element binding protein (pCREB) at Ser133 was observed. Compared to pharmacological inhibition, genetic inhibition of COX-2 resulted in significant reduction of neural stem cells, cell proliferation, and neuroblast differentiation as well as pCREB levels. These results suggest that COX-2 is part of the molecular machinery that regulates neural stem cells, cell proliferation, and neuroblast differentiation during adult hippocampal neurogenesis via pCREB. Additionally, genetic inhibition of COX-2 strongly reduced neural stem cell populations, cell proliferation, and neuroblast differentiation in the dentate gyrus compared to pharmacological inhibition.
Keyword
MeSH Terms
-
Animals
Celecoxib/*pharmacology
Cell Differentiation/drug effects/physiology
Cell Proliferation/drug effects/physiology
Cyclooxygenase 2/*genetics/metabolism
Cyclooxygenase 2 Inhibitors/*pharmacology
Dentate Gyrus/drug effects/*physiology
Male
Mice
Mice, Knockout
Neural Stem Cells/drug effects/physiology
Neurogenesis/drug effects
Celecoxib
Cyclooxygenase 2
Cyclooxygenase 2 Inhibitors
Figure
Cited by 1 articles
-
Neuronal maturation in the hippocampal dentate gyrus via chronic oral administration of Artemisa annua extract is independent of cyclooxygenase 2 signaling pathway in diet-induced obesity mouse model
Hye Kyung Baek, Pan Soo Kim, Ji Ae Song, Dong-Hwa Choi, Do Eun Kim, Seung Il Oh, Sang-Kyu Park, Sung-Jo Kim, Ki-Duk Song, In Koo Hwang, Hyung Seok Seo, Sun Shin Yi
J Vet Sci. 2017;18(2):119-127. doi: 10.4142/jvs.2017.18.2.119.
Reference
-
1. Aasebø IEJ, Blankvoort S, Tashiro A. Critical maturational period of new neurons in adult dentate gyrus for their involvement in memory formation. Eur J Neurosci. 2011; 33:1094–1100.
Article2. Breder CD, Dewitt D, Kraig RP. Characterization of inducible cyclooxygenase in rat brain. J Comp Neurol. 1995; 355:296–315.
Article3. Chen C, Magee JC, Bazan NG. Cyclooxygenase-2 regulates prostaglandin E2 signaling in hippocampal long-term synaptic plasticity. J Neurophysiol. 2002; 87:2851–2857.
Article4. Doré S, Otsuka T, Mito T, Sugo N, Hand T, Wu L, Hurn PD, Traystman RJ, Andreasson K. Neuronal overexpression of cyclooxygenase-2 increases cerebral infarction. Ann Neurol. 2003; 54:155–162.
Article5. Drachman DB, Frank K, Dykes-Hoberg M, Teismann P, Almer G, Przedborski S, Rothstein JD. Cyclooygenase 2 inhibition protects motor neurons and prolongs survival in a transgenic mouse model of ALS. Ann Neurol. 2002; 52:771–778.
Article6. Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003; 18:2803–2812.
Article7. FitzGerald GA, Loll P. COX in a crystal ball: current status and future promise of prostaglandin research. J Clin Invest. 2001; 107:1335–1337.8. Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Figure 43-51. San Diego: Academic Press;1997.9. Goncalves MB, Williams EJ, Yip P, Yáñez-Muñoz RJ, Williams G, Doherty P. The COX-2 inhibitors, meloxicam and nimesulide, suppress neurogenesis in the adult mouse brain. Br J Pharmacol. 2010; 159:1118–1125.
Article10. Hawkey CJ. COX-2 inhibitors. Lancet. 1999; 353:307–314.
Article11. Hoozemans JJM, Rozemuller AJM, Janssen I, De Groot CJA, Veerhuis R, Eikelenboom P. Cyclooxygenase expression in microglia and neurons in Alzheimer's disease and control brain. Acta Neuropathol. 2001; 101:2–8.
Article12. Hwang IK, Yi SS, Yoo KY, Park OK, Yan B, Kim IY, Kim YN, Song W, Moon SM, Won MH, Seong JK, Yoon YS. Effects of treadmill exercise on cyclooxygenase-2 in the hippocampus in type 2 diabetic rats: correlation with the neuroblasts. Brain Res. 2010; 1341:84–92.
Article13. Jung KH, Chu K, Lee ST, Kim J, Sinn DI, Kim JM, Park DK, Lee JJ, Kim SU, Kim M, Lee SK, Roh JK. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol Dis. 2006; 23:237–246.
Article14. Kaltschmidt B, Ndiaye D, Korte M, Pothion S, Arbibe L, Prüllage M, Pfeiffer J, Lindecke A, Staiger V, Israël A, Kaltschmidt C, Mémet S. NF-κB regulates spatial memory formation and synaptic plasticity through protein kinase A/CREB signaling. Mol Cell Biol. 2006; 26:2936–2946.
Article15. Kang KB, Wang TT, Woon CT, Cheah ES, Moore XL, Zhu C, Wong MC. Enhancement of glioblastoma radioresponse by a selective COX-2 inhibitor celecoxib: inhibition of tumor angiogenesis with extensive tumor necrosis. Int J Radiat Oncol Biol Phys. 2007; 67:888–896.
Article16. Kaufmann WE, Worley PF, Taylor CV, Bremer M, Isakson PC. Cyclooxygenase-2 expression during rat neocortical development and in Rett syndrome. Brain Dev. 1997; 19:25–34.
Article17. Kyrkanides S, Moore AH, Olschowka JA, Daeschner JC, Williams JP, Hansen JT, O'Banion MK. Cyclooxygenase-2 modulates brain inflammation-related gene expression in central nervous system radiation injury. Brain Res Mol Brain Res. 2002; 104:159–169.
Article18. Langenbach R, Loftin C, Lee C, Tiano H. Cyclooxygenase knockout mice: models for elucidating isoform-specific functions. Biochem Pharmacol. 1999; 58:1237–1246.19. Lee B, Dziema H, Lee KH, Choi YS, Obrietan K. CRE-mediated transcription and COX-2 expression in the pilocarpine model of status epilepticus. Neurobiol Dis. 2007; 25:80–91.
Article20. Malleret G, Alarcon JM, Martel G, Takizawa S, Vronskaya S, Yin D, Chen IZ, Kandel ER, Shumyatsky GP. Bidirectional regulation of hippocampal long-term synaptic plasticity and its influence on opposing forms of memory. J Neurosci. 2010; 30:3813–3825.
Article21. Miyamoto E. Molecular mechanism of neuronal plasticity: induction and maintenance of long-term potentiation in the hippocampus. J Pharmacol Sci. 2006; 100:433–442.
Article22. Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003; 302:1760–1765.
Article23. Moore AN, Waxham MN, Dash PK. Neuronal activity increases the phosphorylation of the transcription factor cAMP response element-binding protein (CREB) in rat hippocampus and cortex. J Biol Chem. 1996; 271:14214–14220.
Article24. Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, Mahler JF, Kluckman KD, Ledford A, Lee CA, Smithies O. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995; 83:473–482.
Article25. Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, Zhang YJ, Nestler EJ, Duman RS. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. J Neurosci. 2002; 22:3673–3682.
Article26. Nam SM, Kim JW, Yoo DY, Choi JH, Kim W, Jung HY, Won MH, Hwang IK, Seong JK, Yoon YS. Effects of treadmill exercise on neural stem cells, cell proliferation, and neuroblast differentiation in the subgranular zone of the dentate gyrus in cyclooxygenase-2 knockout mice. Neurochem Res. 2013; 38:2559–2569.
Article27. Paulson SK, Zhang JY, Breau AP, Hribar JD, Liu NWK, Jessen SM, Lawal YM, Cogburn JN, Gresk CJ, Markos CS, Maziasz TJ, Schoenhard GL, Burton EG. Pharmacokinetics, tissue distribution, metabolism, and excretion of celecoxib in rats. Drug Metab Dispos. 2000; 28:514–521.28. Pinnock SB, Blake AM, Platt NJ, Herbert J. The roles of BDNF, pCREB and Wnt3a in the latent period preceding activation of progenitor cell mitosis in the adult dentate gyrus by fluoxetine. PLoS One. 2010; 5:e13652.
Article29. Rall JM, Mach SA, Dash PK. Intrahippocampal infusion of a cyclooxygenase-2 inhibitor attenuates memory acquisition in rats. Brain Res. 2003; 968:273–276.
Article30. Rettori V, Gimeno M, Lyson K, McCann SM. Nitric oxide mediates norepinephrine-induced prostaglandin E2 release from the hypothalamus. Proc Natl Acad Sci U S A. 1992; 89:11543–11546.
Article31. Roberson ED, English JD, Adams JP, Selcher JC, Kondratick C, Sweatt JD. The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J Neurosci. 1999; 19:4337–4348.
Article32. Rolando C, Taylor V. Neural stem cell of the hippocampus: development, physiology regulation, and dysfunction in disease. Curr Top Dev Biol. 2014; 107:183–206.33. Russo I, Amornphimoltham P, Weigert R, Barlati S, Bosetti F. Cyclooxygenase-1 is involved in the inhibition of hippocampal neurogenesis after lipopolysaccharide-induced neuroinflammation. Cell Cycle. 2011; 10:2568–2573.
Article34. Sasaki T, Kitagawa K, Sugiura S, Omura-Matsuoka E, Tanaka S, Yagita Y, Okano H, Matsumoto M, Hori M. Implication of cyclooxygenase-2 on enhanced proliferation of neural progenitor cells in the adult mouse hippocampus after ischemia. J Neurosci Res. 2003; 72:461–471.
Article35. Serrano GE, Lelutiu N, Rojas A, Cochi S, Shaw R, Makinson CD, Wang D, FitzGerald GA, Dingledine R. Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus. J Neurosci. 2011; 31:14850–14860.
Article36. Sharifzadeh M, Tavasoli M, Soodi M, Mohammadi-Eraghi S, Ghahremani MH, Roghani A. A time course analysis of cyclooxygenase-2 suggests a role in spatial memory retrieval in rats. Neurosci Res. 2006; 54:171–179.
Article37. Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004; 56:387–437.
Article38. Teather LA, Packard MG, Bazan NG. Post-training cyclooxygenase-2 (COX-2) inhibition impairs memory consolidation. Learn Mem. 2002; 9:41–47.
Article39. Yamamoto K, Asano K, Ito Y, Matsukawa N, Kim S, Yamatodani A. Involvement of hypothalamic cyclooxygenase-2, interleukin-1β and melanocortin in the development of docetaxel-induced anorexia in rats. Toxicology. 2012; 302:190–196.
Article40. Yang H, Zhang J, Andreasson K, Chen C. COX-2 oxidative metabolism of endocannabinoids augments hippocampal synaptic plasticity. Mol Cell Neurosci. 2008; 37:682–695.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neurogenesis and Psychiatry: Focusing on Mood Disorders
- Hippocampal Neurogenesis and Phenotypic Differentiation after Pilocarpine-Induced Seizures in Young Mice
- Repeated restraint stress promotes hippocampal neuronal cell ciliogenesis and proliferation in mice
- Comparison of Neurite Outgrowth Induced by Erythropoietin (EPO) and Carbamylated Erythropoietin (CEPO) in Hippocampal Neural Progenitor Cells
- Temporal Change of Calbindin-D28k Immunoreactivity in the Dentate Gyrus of Voluntary Running Mouse