Korean J Physiol Pharmacol.
2012 Aug;16(4):281-285. 10.4196/kjpp.2012.16.4.281.
Comparison of Neurite Outgrowth Induced by Erythropoietin (EPO) and Carbamylated Erythropoietin (CEPO) in Hippocampal Neural Progenitor Cells
- Affiliations
-
- 1Department of Psychiatry, College of Medicine and Institute of Mental Health, Hanyang University, Seoul 133-791, Korea.
- 2Department of Biochemistry and Molecular Biology, College of Medicine, Hanyang University, Seoul 133-791, Korea. hyeonson@hanyang.ac.kr
- KMID: 2011163
- DOI: http://doi.org/10.4196/kjpp.2012.16.4.281
Abstract
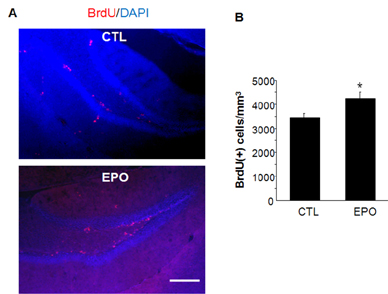
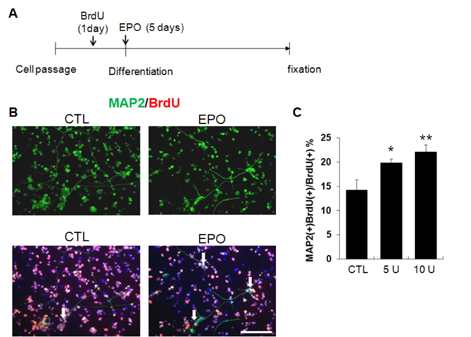
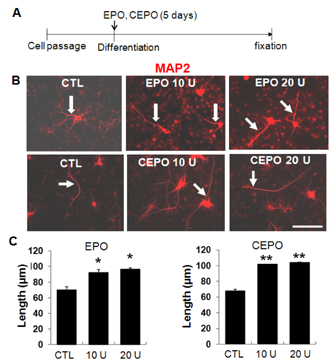
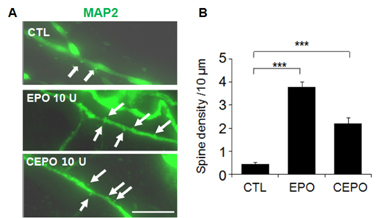
- A previous animal study has shown the effects of erythropoietin (EPO) and its non-erythropoietic carbamylated derivative (CEPO) on neurogenesis in the dentate gyrus. In the present study, we sought to investigate the effect of EPO on adult hippocampal neurogenesis, and to compare the ability of EPO and CEPO promoting dendrite elongation in cultured hippocampal neural progenitor cells. Two-month-old male BALB/c mice were given daily injections of EPO (5 U/g) for seven days and were sacrificed 12 hours after the final injection. Proliferation assays demonstrated that EPO treatment increased the density of bromodeoxyuridine (BrdU)-labeled cells in the subgranular zone (SGZ) compared to that in vehicle-treated controls. Functional differentiation studies using dissociated hippocampal cultures revealed that EPO treatment also increased the number of double-labeled BrdU/microtubule-associated protein 2 (MAP2) neurons compared to those in vehicle-treated controls. Both EPO and CEPO treatment significantly increased the length of neurites and spine density in MAP2(+) cells. In summary, these results provide evidences that EPO and CEPO promote adult hippocampal neurogenesis and neuronal differentiation. These suggest that EPO and CEPO could be a good candidate for treating neuropsychiatric disorders such as depression and anxiety associated with neuronal atrophy and reduced hippocampal neurogenesis.
MeSH Terms
Figure
Reference
-
1. Jelkmann W. Developments in the therapeutic use of erythropoiesis stimulating agents. Br J Haematol. 2008. 141:287–297.2. Sirén AL, Fasshauer T, Bartels C, Ehrenreich H. Therapeutic potential of erythropoietin and its structural or functional variants in the nervous system. Neurotherapeutics. 2009. 6:108–127.3. Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, Savino C, Bianchi M, Nielsen J, Gerwien J, Kallunki P, Larsen AK, Helboe L, Christensen S, Pedersen LO, Nielsen M, Torup L, Sager T, Sfacteria A, Erbayraktar S, Erbayraktar Z, Gokmen N, Yilmaz O, Cerami-hand C, Xie QW, Coleman T, Cerami A, Brines M. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004. 305:239–242.4. Xiong Y, Mahmood A, Zhang Y, Meng Y, Zhang ZG, Qu C, Sager TN, Chopp M. Effects of posttraumatic carbamylated erythropoietin therapy on reducing lesion volume and hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome in rats following traumatic brain injury. J Neurosurg. 2011. 114:549–559.5. David DJ, Wang J, Samuels BA, Rainer Q, David I, Gardier AM, Hen R. Implications of the functional integration of adult-born hippocampal neurons in anxiety-depression disorders. Neuroscientist. 2010. 16:578–591.6. Miskowiak KW, Vinberg M, Harmer CJ, Ehrenreich H, Knudsen GM, Macoveanu J, Hansen AR, Paulson OB, Siebner HR, Kessing LV. Effects of erythropoietin on depressive symptoms and neurocognitive deficits in depression and bipolar disorder. Trials. 2010. 11:97.7. Kirkeby A, Torup L, Bochsen L, Kjalke M, Abel K, Theilgaardmonch K, Johansson PI, Bjørn SE, Gerwien J, Leist M. High-dose erythropoietin alters platelet reactivity and bleeding time in rodents in contrast to the neuroprotective variant carbamyl-erythropoietin (CEPO). Thromb Haemost. 2008. 99:720–728.8. Leconte C, Bihel E, Lepelletier FX, Bouët V, Saulnier R, Petit E, Boulouard M, Bernaudin M, Schumann-bard P. Comparison of the effects of erythropoietin and its carbamylated derivative on behaviour and hippocampal neurogenesis in mice. Neuropharmacology. 2011. 60:354–364.9. Montero M, Poulsen FR, Noraberg J, Kirkeby A, Van BJ, Leist M, Zimmer J. Comparison of neuroprotective effects of erythropoietin (EPO) and carbamylerythropoietin (CEPO) against ischemia-like oxygen-glucose deprivation (OGD) and NMDA excitotoxicity in mouse hippocampal slice cultures. Exp Neurol. 2007. 204:106–117.10. Ransome MI, Turnley AM. Erythropoietin promotes axonal growth in a model of neuronal polarization. Mol Cell Neurosci. 2008. 38:537–547.11. Wang L, Zhang ZG, Gregg SR, Zhang RL, Jiao Z, Letourneau Y, Liu X, Feng Y, Gerwien J, Torup L, Leist M, Noguchi CT, Chen ZY, Chopp M. The Sonic hedgehog pathway mediates carbamylated erythropoietin-enhanced proliferation and differentiation of adult neural progenitor cells. J Biol Chem. 2007. 282:32462–32470.12. Son H, Yu IT, Hwang SJ, Kim JS, Lee SH, Lee YS, Kaang BK. Lithium enhances long-term potentiation independently of hippocampal neurogenesis in the rat dentate gyrus. J Neurochem. 2003. 85:872–881.13. Amoureux MC, Cunningham BA, Edelman GM, Crossin KL. N-CAM binding inhibits the proliferation of hippocampal progenitor cells and promotes their differentiation to a neuronal phenotype. J Neurosci. 2000. 20:3631–3640.14. Johe KK, Hazel TG, Muller T, Dugich-djordjevic MM, Mckay RD. Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev. 1996. 10:3129–3140.15. Kim JS, Chang MY, Yu IT, Kim JH, Lee SH, Lee YS, Son H. Lithium selectively increases neuronal differentiation of hippocampal neural progenitor cells both in vitro and in vivo. J Neurochem. 2004. 89:324–336.16. Hörkkö S, Savolainen MJ, Kervinen K, Kesäniemi YA. Carbamylation-induced alterations in low-density lipoprotein metabolism. Kidney Int. 1992. 41:1175–1181.17. Ransome MI, Turnley AM. Systemically delivered Erythropoietin transiently enhances adult hippocampal neurogenesis. J Neurochem. 2007. 102:1953–1965.18. Kretz A, Happold CJ, Marticke JK, Isenmann S. Erythropoietin promotes regeneration of adult CNS neurons via Jak2/Stat3 and PI3K/AKT pathway activation. Mol Cell Neurosci. 2005. 29:569–579.19. Noguchi CT, Asavaritikrai P, Teng R, Jia Y. Role of erythropoietin in the brain. Crit Rev Oncol Hematol. 2007. 64:159–171.20. van der Kooij MA, Groenendaal F, Kavelaars A, Heijnen CJ, Van BF. Neuroprotective properties and mechanisms of erythropoietin in in vitro and in vivo experimental models for hypoxia/ischemia. Brain Res Rev. 2008. 59:22–33.21. Wang L, Zhang ZG, Gregg SR, Zhang RL, Jiao Z, Letourneau Y, Liu X, Feng Y, Gerwien J, Torup L, Leist M, Noguchi CT, Chen ZY, Chopp M. The Sonic hedgehog pathway mediates carbamylated erythropoietin-enhanced proliferation and differentiation of adult neural progenitor cells. J Biol Chem. 2007. 282:32462–32470.22. Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010. 11:339–350.23. Lapchak PA. Carbamylated erythropoietin to treat neuronal injury: new development strategies. Expert Opin Investig Drugs. 2008. 17:1175–1186.24. Kim SS, Lee KH, Sung DK, Shim JW, Kim MJ, Jeon GW, Chang YS, Park WS. Erythropoietin attenuates brain injury, subventricular zone expansion, and sensorimotor deficits in hypoxic-ischemic neonatal rats. J Korean Med Sci. 2008. 23:484–491.25. Ehrenreich H, Degner D, Meller J, Brines M, Béhé M, Hasselblatt M, Woldt H, Falkai P, Knerlich F, Jacob S, Von AN, Maier W, Brück W, Rüther E, Cerami A, Becker W, Sirén AL. Erythropoietin: a candidate compound for neuroprotection in schizophrenia. Mol Psychiatry. 2004. 9:42–54.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Serotonin on Erythropoietin Expression in Mouse Hippocampus
- Effects of Erythropoietin in Hypoxia-Induced Ischemia on Differentiated Human Neuroblastoma SH-SY5Y and Rat Stroke Model
- Effect of Erythropoietin on the Production of Nitric Oxide in Trabecular Meshwork Cells
- Systemic injection of recombinant human erythropoietin after focal cerebral ischemia enhances oligodendroglial and endothelial progenitor cells in rat brain
- Expression Patterns of Erythropoietin and Erythropoietin Receptor in the Spiral Ganglion of Guinea Pig after Noise Exposure