Immune Netw.
2016 Dec;16(6):373-380. 10.4110/in.2016.16.6.373.
Peroxiredoxin-3 Is Involved in Bactericidal Activity through the Regulation of Mitochondrial Reactive Oxygen Species
- Affiliations
-
- 1Department of Molecular Cell Biology and Samsung Biomedical Research Institute, Sungkyunkwan University School of Medicine, Suwon 16419, Korea. thylee@skku.edu
- KMID: 2362802
- DOI: http://doi.org/10.4110/in.2016.16.6.373
Abstract
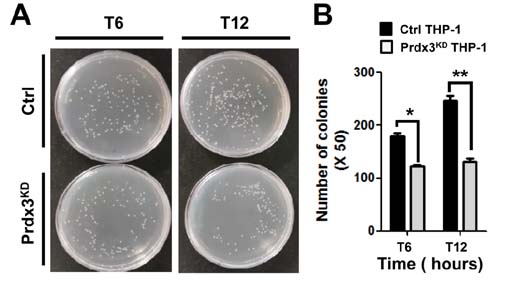
- Peroxiredoxin-3 (Prdx3) is a mitochondrial protein of the thioredoxin family of antioxidant peroxidases and is the principal peroxidase responsible for metabolizing mitochondrial hydrogen peroxide. Recent reports have shown that mitochondrial reactive oxygen species (mROS) contribute to macrophage-mediated bactericidal activity in response to Toll-like receptors. Herein, we investigated the functional effect of Prdx3 in bactericidal activity. The mitochondrial localization of Prdx3 in HEK293T cells was confirmed by cell fractionation and confocal microscopy analyses. To investigate the functional role of Prdx3 in bactericidal activity, Prdx3-knockdown (Prdx3(KD)) THP-1 cells were generated. The mROS levels in Prdx3(KD) THP-1 cells were significantly higher than those in control THP-1 cells. Moreover, the mROS levels were markedly increased in response to lipopolysaccharide. Notably, the Salmonella enterica serovar Typhimurium infection assay revealed that the Prdx3(KD) THP-1 cells were significantly resistant to S. Typhimurium infection, as compared with control THP-1 cells. Taken together, these results indicate that Prdx3 is functionally important in bactericidal activity through the regulation of mROS.
Keyword
MeSH Terms
-
Cell Fractionation
Humans
Hydrogen Peroxide
Lipopolysaccharides
Microscopy, Confocal
Mitochondrial Proteins
Peroxidase
Peroxidases
Reactive Oxygen Species*
Salmonella enterica
Serogroup
Thioredoxins
Toll-Like Receptors
Hydrogen Peroxide
Lipopolysaccharides
Mitochondrial Proteins
Peroxidase
Peroxidases
Reactive Oxygen Species
Thioredoxins
Toll-Like Receptors
Figure
Cited by 1 articles
-
p62 Negatively Regulates TLR4 Signaling via Functional Regulation of the TRAF6-ECSIT Complex
Mi-Jeong Kim, Yoon Min, Jeongho Kwon, Juhee Son, Ji Seon Im, Jaekyoon Shin, Ki-Young Lee
Immune Netw. 2019;19(3):. doi: 10.4110/in.2019.19.e16.
Reference
-
1. Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009; 417:1–13.
Article2. Mailloux RJ. Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol. 2015; 4:381–398.
Article3. Mailloux RJ, Jin X, Willmore WG. Redox regulation of mitochondrial function with emphasis on cysteine oxidation reactions. Redox Biol. 2014; 2:123–139.
Article4. Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002; 80:780–787.
Article5. Titov DV, Cracan V, Goodman RP, Peng J, Grabarek Z, Mootha VK. Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio. Science. 2016; 352:231–235.
Article6. Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell. 2012; 48:158–167.
Article7. Tang X, Luo YX, Chen HZ, Liu DP. Mitochondria, endothelial cell function, and vascular diseases. Front Physiol. 2014; 5:175.
Article8. Li X, Fang P, Li Y, Kuo YM, Andrews AJ, Nanayakkara G, Johnson C, Fu H, Shan H, Du F, Hoffman NE, Yu D, Eguchi S, Madesh M, Koch WJ, Sun J, Jiang X, Wang H, Yang X. Mitochondrial reactive oxygen species mediate lysophosphatidylcholine-induced endothelial cell activation. Arterioscler Thromb Vasc Biol. 2016; 36:1090–1100.
Article9. Finkel T. Signal transduction by mitochondrial oxidants. J Biol Chem. 2012; 287:4434–4440.
Article10. West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011; 472:476–480.
Article11. Geng J, Sun X, Wang P, Zhang S, Wang X, Wu H, Hong L, Xie C, Li X, Zhao H, Liu Q, Jiang M, Chen Q, Zhang J, Li Y, Song S, Wang HR, Zhou R, Johnson RL, Chien KY, Lin SC, Han J, Avruch J, Chen L, Zhou D. Kinases Mst1 and Mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity. Nat Immunol. 2015; 16:1142–1152.
Article12. Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996; 86:147–157.
Article13. Susin SA, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, Daugas E, Geuskens M, Kroemer G. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med. 1996; 184:1331–1341.
Article14. Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012; 5:9–19.
Article15. Fisher AB. Peroxiredoxin 6: a bifunctional enzyme with glutathione peroxidase and phospholipase A(2) activities. Antioxid Redox Signal. 2011; 15:831–844.
Article16. Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003; 300:650–653.
Article17. Park MH, Jo M, Kim YR, Lee CK, Hong JT. Roles of peroxiredoxins in cancer, neurodegenerative diseases and inflammatory diseases. Pharmacol Ther. 2016; 163:1–23.
Article18. Fatma N, Kubo E, Sharma P, Beier DR, Singh DP. Impaired homeostasis and phenotypic abnormalities in Prdx6-/-mice lens epithelial cells by reactive oxygen species: increased expression and activation of TGFbeta. Cell Death Differ. 2005; 12:734–750.
Article19. Chang TS, Cho CS, Park S, Yu S, Kang SW, Rhee SG. Peroxiredoxin III, a mitochondrion-specific peroxidase, regulates apoptotic signaling by mitochondria. J Biol Chem. 2004; 279:41975–41984.
Article20. Granville DJ, Gottlieb RA. Mitochondria: regulators of cell death and survival. Scientific World Journal. 2002; 2:1569–1578.
Article21. Cunniff B. Peroxiredoxin 3 levels regulate a mitochondrial redox setpoint in malignant mesothelioma cells. Redox Biol. 2014; 3:79–87.
Article22. Li L, Yu AQ. The functional role of peroxiredoxin 3 in reactive oxygen species, apoptosis, and chemoresistance of cancer cells. J Cancer Res Clin Oncol. 2015; 141:2071–2077.
Article23. Mi WS, Park J, Shim JH, Chun E, Lee KY. Ubiquitination of ECSIT is crucial for the activation of p65/p50 NF-kappaBs in Toll-like receptor 4 signaling. Mol Biol Cell. 2015; 26:151–160.24. Wi SM, Moon G, Kim J, Kim ST, Shim JH, Chun E, Lee KY. TAK1-ECSIT-TRAF6 complex plays a key role in the TLR4 signal to activate NF-kappaB. J Biol Chem. 2014; 289:35205–35214.
Article25. Moon G, Kim J, Min Y, Wi SM, Shim JH, Chun E, Lee KY. Phosphoinositide-dependent kinase-1 inhibits TRAF6 ubiquitination by interrupting the formation of TAK1-TAB2 complex in TLR4 signaling. Cell Signal. 2015; 27:2524–2533.
Article26. Kim SY, Baik KH, Baek KH, Chah KH, Kim KA, Moon G, Jung E, Kim ST, Shim JH, Greenblatt MB, Chun E, Lee KY. S6K1 negatively regulates TAK1 activity in the toll-like receptor signaling pathway. Mol Cell Biol. 2014; 34:510–521.
Article27. Yong KS, Jeong S, Chah KH, Jung E, Baek KH, Kim ST, Shim JH, Chun E, Lee KY. Salt-inducible kinases 1 and 3 negatively regulate Toll-like receptor 4-mediated signal. Mol Endocrinol. 2013; 27:1958–1968.
Article28. Kim SY, Jeong S, Jung E, Baik KH, Chang MH, Kim SA, Shim JH, Chun E, Lee KY. AMP-activated protein kinase-alpha1 as an activating kinase of TGF-beta-activated kinase 1 has a key role in inflammatory signals. Cell Death Dis. 2012; 3:e357.29. Wi SM, Lee KY. 5-aminoimidazole-4-carboxamide riboside induces apoptosis through AMP-activated protein kinase-independent and NADPH oxidase-dependent pathways. Immune Netw. 2014; 14:241–248.
Article30. Kopp E, Medzhitov R, Carothers J, Xiao C, Douglas I, Janeway CA, Ghosh S. ECSIT is an evolutionarily conserved intermediate in the Toll/IL-1 signal transduction pathway. Genes Dev. 1999; 13:2059–2071.
Article