J Periodontal Implant Sci.
2015 Dec;45(6):238-246. 10.5051/jpis.2015.45.6.238.
Bone formation around rhBMP-2-coated implants in rabbit sinuses with or without absorbable collagen sponge grafting
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. drjew@yuhs.ac
- 2Department of Periodontology, Kyung Hee University School of Dentistry, Seoul, Korea.
- KMID: 2354942
- DOI: http://doi.org/10.5051/jpis.2015.45.6.238
Abstract
- PURPOSE
The purpose of this study was to evaluate bone formation around recombinant human bone morphogenetic protein (rhBMP-2)-coated implants placed with or without absorbable collagen sponge (ACS) in rabbit maxillary sinuses.
METHODS
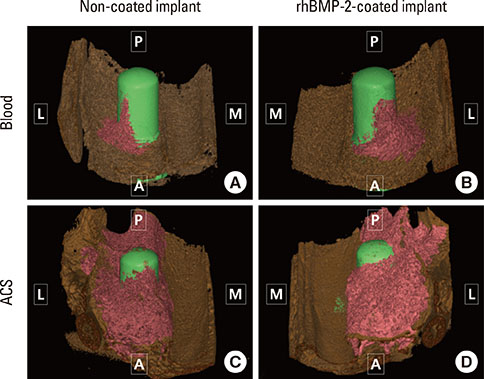
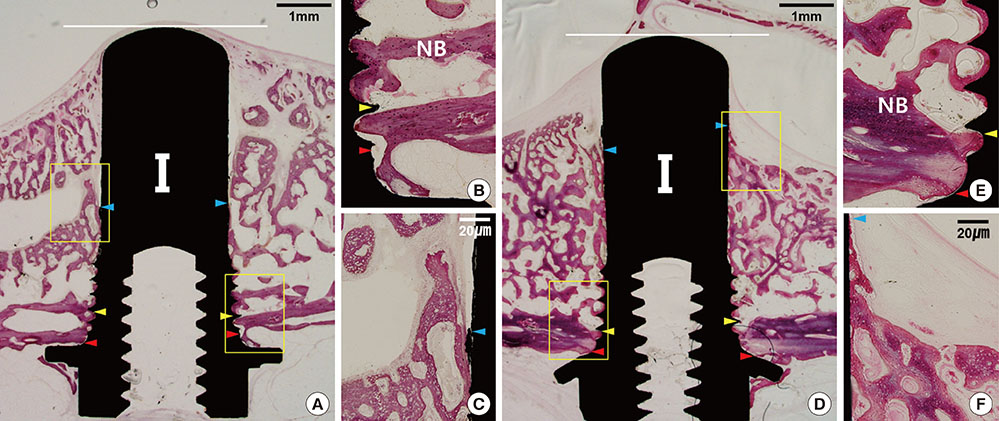
The Schneiderian membrane was elevated and an implant was placed in 24 sinuses in 12 rabbits. The space created beneath the elevated membrane was filled with either blood (n=6) or ACS (n=6). In the rabbits in which this space was filled with blood, rhBMP-2-coated and non-coated implants were alternately placed on different sides. The resulting groups were referred to as the BC and BN groups, respectively. The AC and AN groups were produced in ACS-grafted rabbits in the same manner. Radiographic and histomorphometric analyses were performed after eight weeks of healing.
RESULTS
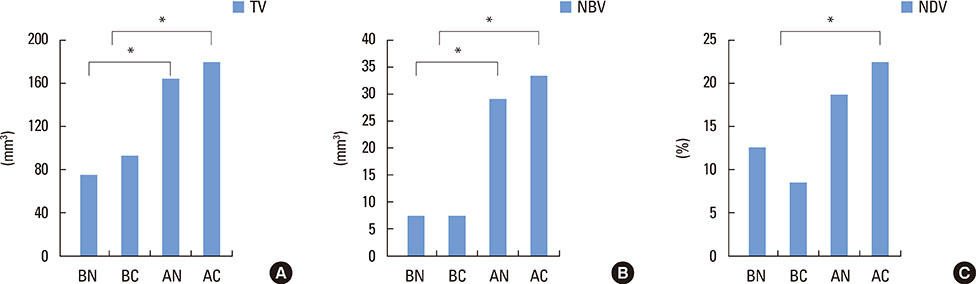
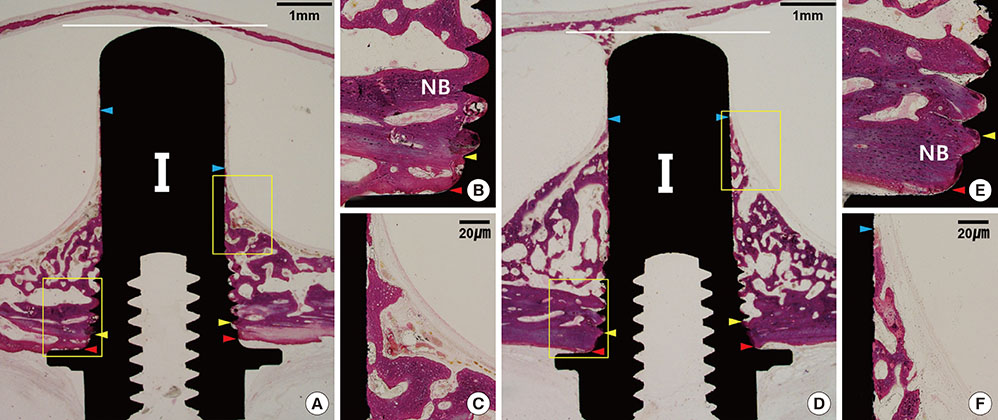
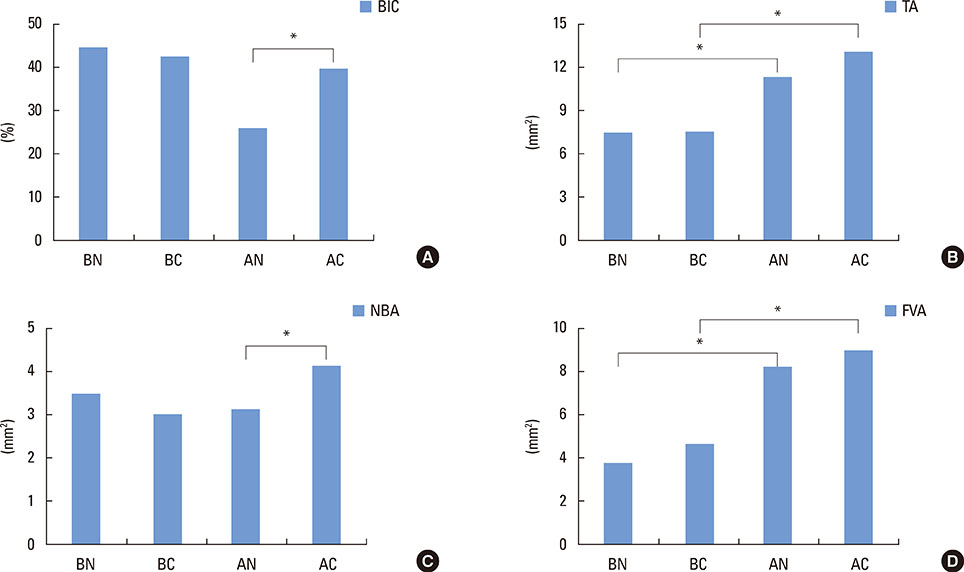
In micro-computed tomography analysis, the total augmented volume and new bone volume were significantly greater in the ACS-grafted sinuses than in the blood-filled sinuses (P<0.05). The histometric analysis showed that the areas of new bone and bone-to-implant contact were significantly larger in the AC group than in the AN group (P<0.05). In contrast, none of the parameters differed significantly between the BC and BN groups.
CONCLUSIONS
The results of this pilot study indicate that the insertion of ACS after elevating the Schneiderian membrane, simultaneously with implant placement, can significantly increase the volume of the augmentation. However, in the present study, the rhBMP-2 coating exhibited limited effectiveness in enhancing the quantity and quality of regenerated bone.
Keyword
MeSH Terms
Figure
Reference
-
1. Del Fabbro M, Corbella S, Weinstein T, Ceresoli V, Taschieri S. Implant survival rates after osteotome-mediated maxillary sinus augmentation: a systematic review. Clin Implant Dent Relat Res. 2012; 14:Suppl 1. e159–68.
Article2. Browaeys H, Bouvry P, De Bruyn H. A literature review on biomaterials in sinus augmentation procedures. Clin Implant Dent Relat Res. 2007; 9:166–243.
Article3. Araújo M, Linder E, Lindhe J. Effect of a xenograft on early bone formation in extraction sockets: an experimental study in dog. Clin Oral Implants Res. 2009; 20:1–6.
Article4. Shanbhag S, Shanbhag V, Stavropoulos A. Volume changes of maxillary sinus augmentations over time: a systematic review. Int J Oral Maxillofac Implants. 2014; 29:881–973.
Article5. Lee J, Susin C, Rodriguez NA, de Stefano J, Prasad HS, Buxton AN, et al. Sinus augmentation using rhBMP-2/ACS in a mini-pig model: relative efficacy of autogenous fresh particulate iliac bone grafts. Clin Oral Implants Res. 2013; 24:497–504.
Article6. Yamada Y, Nakamura S, Ueda M, Ito K. Osteotome technique with injectable tissue-engineered bone and simultaneous implant placement by cell therapy. Clin Oral Implants Res. 2013; 24:468–542.
Article7. Lundgren S, Cricchio G, Palma VC, Salata LA, Sennerby L. Sinus membrane elevation and simultaneous insertion of dental implants: a new surgical technique in maxillary sinus floor augmentation. Periodontol 2000. 2008; 47:193–205.
Article8. Lundgren S, Andersson S, Gualini F, Sennerby L. Bone reformation with sinus membrane elevation: a new surgical technique for maxillary sinus floor augmentation. Clin Implant Dent Relat Res. 2004; 6:165–238.
Article9. Palma VC, Magro-Filho O, de Oliveria JA, Lundgren S, Salata LA, Sennerby L. Bone reformation and implant integration following maxillary sinus membrane elevation: an experimental study in primates. Clin Implant Dent Relat Res. 2006; 8:11–24.
Article10. Cricchio G, Sennerby L, Lundgren S. Sinus bone formation and implant survival after sinus membrane elevation and implant placement: a 1- to 6-year follow-up study. Clin Oral Implants Res. 2011; 22:1200–1212.
Article11. Ellegaard B, Baelum V, Kølsen-Petersen J. Non-grafted sinus implants in periodontally compromised patients: a time-to-event analysis. Clin Oral Implants Res. 2006; 17:156–220.
Article12. Thor A, Sennerby L, Hirsch JM, Rasmusson L. Bone formation at the maxillary sinus floor following simultaneous elevation of the mucosal lining and implant installation without graft material: an evaluation of 20 patients treated with 44 Astra Tech implants. J Oral Maxillofac Surg. 2007; 65:Suppl 1. 64–72.
Article13. Cricchio G, Imburgia M, Sennerby L, Lundgren S. Immediate loading of implants placed simultaneously with sinus membrane elevation in the posterior atrophic maxilla: a two-year follow-up study on 10 patients. Clin Implant Dent Relat Res. 2014; 16:609–626.
Article14. Pinchasov G, Juodzbalys G. Graft-free sinus augmentation procedure: a literature review. J Oral Maxillofac Res. 2014; 5:e1.
Article15. Hatano N, Sennerby L, Lundgren S. Maxillary sinus augmentation using sinus membrane elevation and peripheral venous blood for implant-supported rehabilitation of the atrophic posterior maxilla: case series. Clin Implant Dent Relat Res. 2007; 9:150–155.
Article16. Iida S, Tanaka N, Kogo M, Matsuya T. Migration of a dental implant into the maxillary sinus. A case report. Int J Oral Maxillofac Surg. 2000; 29:358–367.
Article17. Raghoebar GM, van Weissenbruch R, Vissink A. Rhino-sinusitis related to endosseous implants extending into the nasal cavity. A case report. Int J Oral Maxillofac Surg. 2004; 33:312–316.18. Cole BJ, Bostrom MP, Pritchard TL, Sumner DR, Tomin E, Lane JM, et al. Use of bone morphogenetic protein 2 on ectopic porous coated implants in the rat. Clin Orthop Relat Res. 1997; (345):219–247.
Article19. Schmidmaier G, Wildemann B, Cromme F, Kandziora F, Haas NP, Raschke M. Bone morphogenetic protein-2 coating of titanium implants increases biomechanical strength and accelerates bone remodeling in fracture treatment: a biomechanical and histological study in rats. Bone. 2002; 30:816–838.
Article20. Hall J, Sorensen RG, Wozney JM, Wikesjö UM. Bone formation at rhBMP-2-coated titanium implants in the rat ectopic model. J Clin Periodontol. 2007; 34:444–495.
Article21. Wikesjö UM, Qahash M, Polimeni G, Susin C, Shanaman RH, Rohrer MD, et al. Alveolar ridge augmentation using implants coated with recombinant human bone morphogenetic protein-2: histologic observations. J Clin Periodontol. 2008; 35:1001–1011.
Article22. Pelaez M, Susin C, Lee J, Fiorini T, Bisch FC, Dixon DR, et al. Effect of rhBMP-2 dose on bone formation/maturation in a rat critical-size calvarial defect model. J Clin Periodontol. 2014; 41:827–863.
Article23. Choi Y, Yun JH, Kim CS, Choi SH, Chai JK, Jung UW. Sinus augmentation using absorbable collagen sponge loaded with Escherichia coli-expressed recombinant human bone morphogenetic protein 2 in a standardized rabbit sinus model: a radiographic and histologic analysis. Clin Oral Implants Res. 2012; 23:682–691.24. Hong JY, Kim MS, Lim HC, Lee JS, Choi SH, Jung UW. A high concentration of recombinant human bone morphogenetic protein-2 induces low-efficacy bone regeneration in sinus augmentation: a histomorphometric analysis in rabbits. Clin Oral Implants Res. 2015.
Article25. Leknes KN, Yang J, Qahash M, Polimeni G, Susin C, Wikesjö UM. Alveolar ridge augmentation using implants coated with recombinant human bone morphogenetic protein-2: radiographic observations. Clin Oral Implants Res. 2008; 19:1027–1060.
Article26. Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010; 25:1468–1554.
Article27. Jung UW, Unursaikhan O, Park JY, Lee JS, Otgonbold J, Choi SH. Tenting effect of the elevated sinus membrane over an implant with adjunctive use of a hydroxyapatite-powdered collagen membrane in rabbits. Clin Oral Implants Res. 2015; 26:663–733.
Article28. Lee SW, Hahn BD, Kang TY, Lee MJ, Choi JY, Kim MK, et al. Hydroxyapatite and collagen combination-coated dental implants display better bone formation in the peri-implant area than the same combination plus bone morphogenetic protein-2-coated implants, hydroxyapatite only coated implants, and uncoated implants. J Oral Maxillofac Surg. 2014; 72:53–60.
Article29. Srouji S, Kizhner T, Ben David D, Riminucci M, Bianco P, Livne E. The Schneiderian membrane contains osteoprogenitor cells: in vivo and in vitro study. Calcif Tissue Int. 2009; 84:138–183.
Article30. Gruber R, Kandler B, Fuerst G, Fischer MB, Watzek G. Porcine sinus mucosa holds cells that respond to bone morphogenetic protein (BMP)-6 and BMP-7 with increased osteogenic differentiation in vitro. Clin Oral Implants Res. 2004; 15:575–655.
Article31. Choi Y, Lee JS, Kim YJ, Kim MS, Choi SH, Cho KS, et al. Recombinant human bone morphogenetic protein-2 stimulates the osteogenic potential of the Schneiderian membrane: a histometric analysis in rabbits. Tissue Eng Part A. 2013; 19:1994–2004.
Article32. Lee J, Decker JF, Polimeni G, Cortella CA, Rohrer MD, Wozney JM, et al. Evaluation of implants coated with rhBMP-2 using two different coating strategies: a critical-size supraalveolar peri-implant defect study in dogs. J Clin Periodontol. 2010; 37:582–672.
Article33. Decker JF, Lee J, Cortella CA, Polimeni G, Rohrer MD, Wozney JM, et al. Evaluation of implants coated with recombinant human bone morphogenetic protein-2 and vacuum-dried using the critical-size supraalveolar peri-implant defect model in dogs. J Periodontol. 2010; 81:1839–1888.
Article34. Kim MS, Kwon JY, Lee JS, Song JS, Choi SH, Jung UW. Low-dose recombinant human bone morphogenetic protein-2 to enhance the osteogenic potential of the Schneiderian membrane in the early healing phase: in vitro and in vivo studies. J Oral Maxillofac Surg. 2014; 72:1480–1574.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Body text. Bone formation around rhBMP-2-coated implants in rabbit sinuses with or without absorbable collagen sponge grafting
- Effects of rhBMP‑2 with various carriers on maxillofacial bone regeneration through computed tomography evaluation
- Bone healing capacity of the collagen bone filler (TERUPLUG(R)) and rhBMP-2 in the rabbit cranium defect
- Tissue Engineering with rhBMP-2: Bone Reconstruction in Implant Dentistry
- Comparative analysis of carrier systems for delivering bone morphogenetic proteins