Diabetes Metab J.
2016 Oct;40(5):396-405. 10.4093/dmj.2016.40.5.396.
Repeated Glucose Deprivation/Reperfusion Induced PC-12 Cell Death through the Involvement of FOXO Transcription Factor
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Onhospital, Busan, Korea.
- 2Division of Endocrinology and Metabolism, Department of Internal Medicine, Inje University College of Medicine, Busan, Korea. kmkdoc@hanmail.net pjhdoc@chol.net
- 3Paik Institute for Clinical Research, Molecular Therapy Lab, Inje University, Busan, Korea.
- KMID: 2354613
- DOI: http://doi.org/10.4093/dmj.2016.40.5.396
Abstract
- BACKGROUND
Cognitive impairment and brain damage in diabetes is suggested to be associated with hypoglycemia. The mechanisms of hypoglycemia-induced neural death and apoptosis are not clear and reperfusion injury may be involved. Recent studies show that glucose deprivation/reperfusion induced more neuronal cell death than glucose deprivation itself. The forkhead box O (FOXO) transcription factors are implicated in the regulation of cell apoptosis and survival, but their role in neuronal cells remains unclear. We examined the role of FOXO transcription factors and the involvement of the phosphatidylinositol 3-kinase (PI3K)/Akt and apoptosis-related signaling pathways in PC-12 cells exposed to repeated glucose deprivation/reperfusion.
METHODS
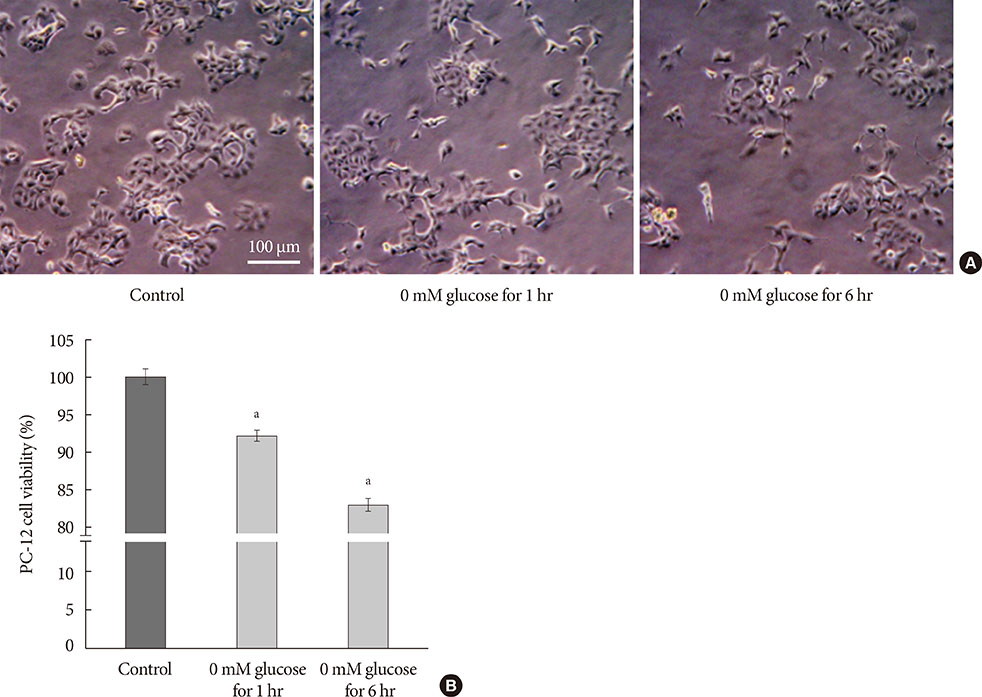
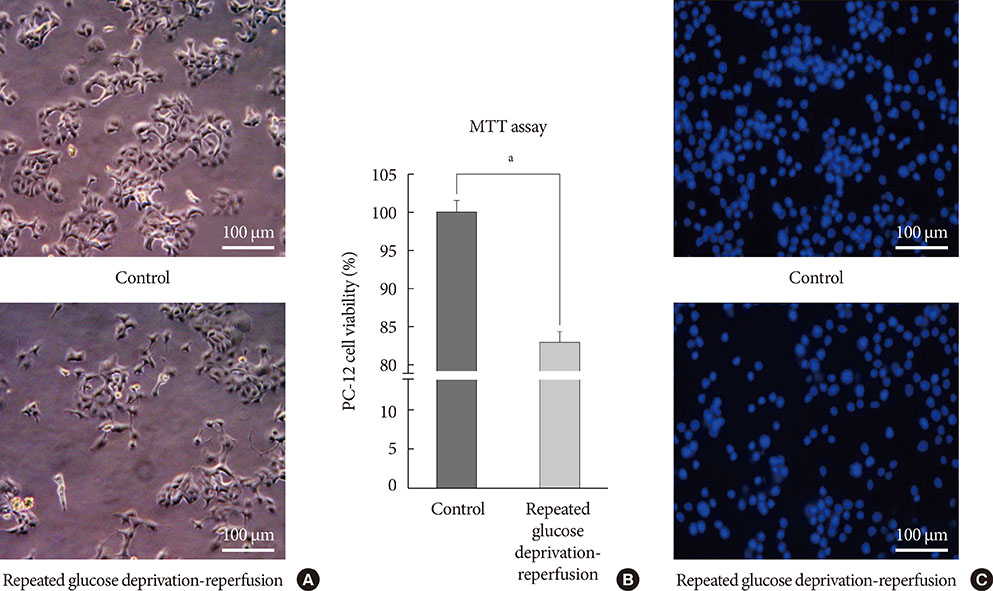
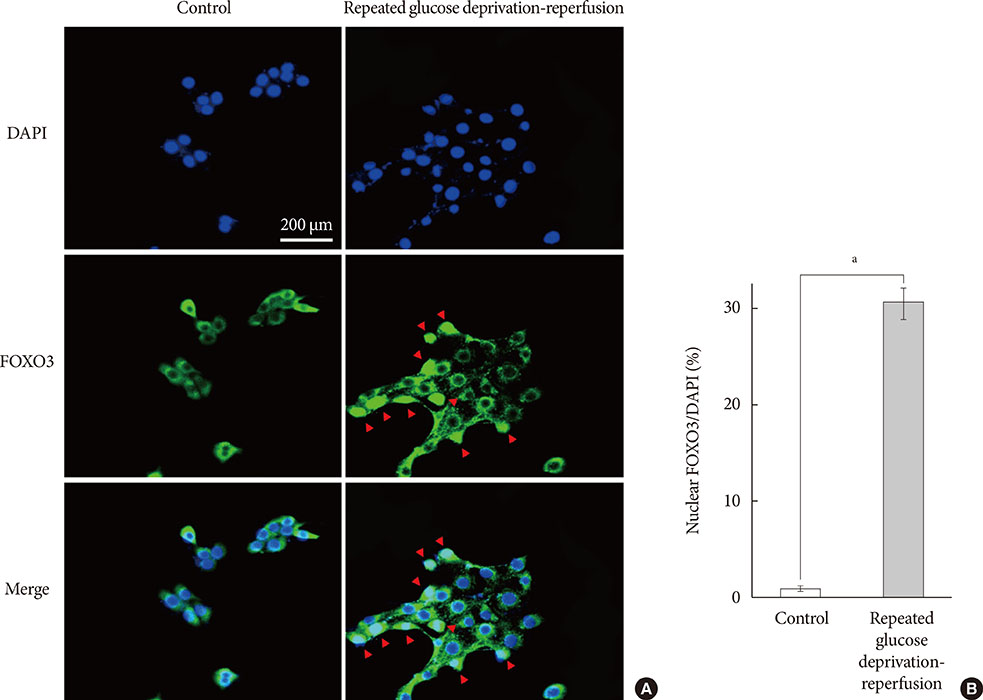
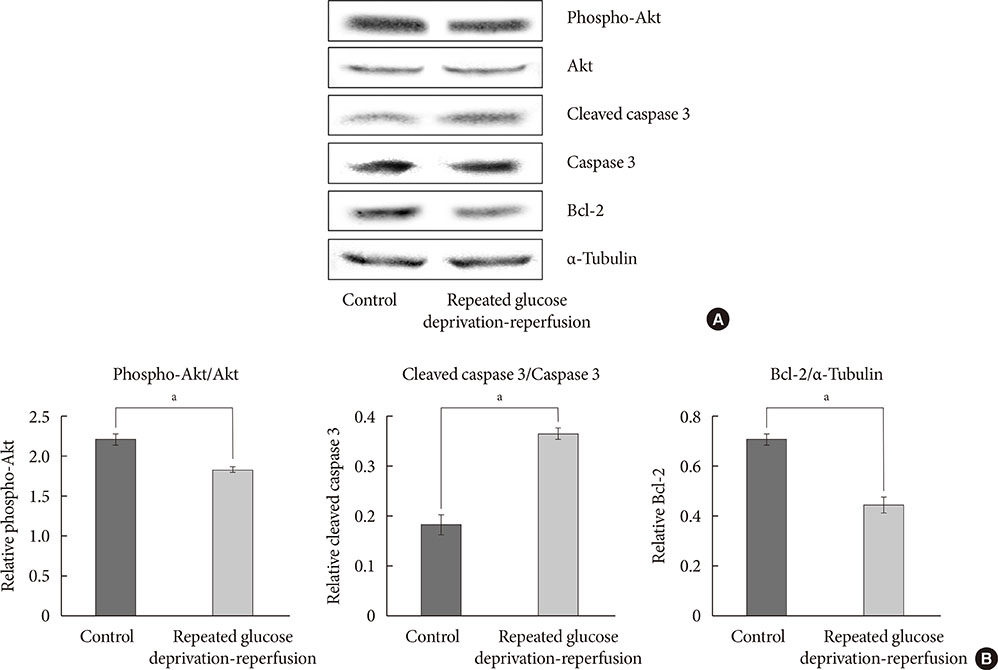
PC-12 cells were exposed to control (Dulbecco's Modified Eagle Medium [DMEM] containing 25 mM glucose) or glucose deprivation/reperfusion (DMEM with 0 mM glucose for 6 hours and then DMEM with 25 mM glucose for 18 hours) for 5 days. MTT assay and Western blot analysis were performed for cell viability, apoptosis, and the expression of survival signaling pathways. FOXO3/4',6-diamidino-2-phenylindole staining was done to ascertain the involvement of FOXO transcription factors in glucose deprivation/reperfusion conditions.
RESULTS
Compared to PC-12 cells not exposed to hypoglycemia, cells exposed to glucose deprivation/reperfusion showed a reduction of cell viability, decreased expression of phosphorylated Akt and Bcl-2, and an increase of cleaved caspase-3 expression. Of note, FOXO3 protein was localized in the nuclei of glucose deprivation/reperfusion cells but not in the control cells.
CONCLUSION
Repeated glucose deprivation/reperfusion caused the neuronal cell death. Activated FOXO3 via the PI3K/Akt pathway in repeated glucose deprivation/reperfusion was involved in genes related to apoptosis.
Keyword
MeSH Terms
Figure
Reference
-
1. Cukierman-Yaffe T, Gerstein HC, Williamson JD, Lazar RM, Lovato L, Miller ME, Coker LH, Murray A, Sullivan MD, Marcovina SM, Launer LJ. Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) Investigators. Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the action to control cardiovascular risk in diabetes-memory in diabetes (ACCORD-MIND) trial. Diabetes Care. 2009; 32:221–226.2. Bruehl H, Wolf OT, Sweat V, Tirsi A, Richardson S, Convit A. Modifiers of cognitive function and brain structure in middle-aged and elderly individuals with type 2 diabetes mellitus. Brain Res. 2009; 1280:186–194.3. Ahtiluoto S, Polvikoski T, Peltonen M, Solomon A, Tuomilehto J, Winblad B, Sulkava R, Kivipelto M. Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology. 2010; 75:1195–1202.4. Whitmer RA, Karter AJ, Yaffe K, Quesenberry CP Jr, Selby JV. Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus. JAMA. 2009; 301:1565–1572.5. Auer RN. Hypoglycemic brain damage. Metab Brain Dis. 2004; 19:169–175.6. Biessels GJ, Staekenborg S, Brunner E, Brayne C, Scheltens P. Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 2006; 5:64–74.7. Fishel MA, Watson GS, Montine TJ, Wang Q, Green PS, Kulstad JJ, Cook DG, Peskind ER, Baker LD, Goldgaber D, Nie W, Asthana S, Plymate SR, Schwartz MW, Craft S. Hyperinsulinemia provokes synchronous increases in central inflammation and beta-amyloid in normal adults. Arch Neurol. 2005; 62:1539–1544.8. Matsuzaki T, Sasaki K, Tanizaki Y, Hata J, Fujimi K, Matsui Y, Sekita A, Suzuki SO, Kanba S, Kiyohara Y, Iwaki T. Insulin resistance is associated with the pathology of Alzheimer disease: the Hisayama study. Neurology. 2010; 75:764–770.9. Yaffe K, Lindquist K, Schwartz AV, Vitartas C, Vittinghoff E, Satterfield S, Simonsick EM, Launer L, Rosano C, Cauley JA, Harris T. Advanced glycation end product level, diabetes, and accelerated cognitive aging. Neurology. 2011; 77:1351–1356.10. Yu S, Liu M, Gu X, Ding F. Neuroprotective effects of salidroside in the PC12 cell model exposed to hypoglycemia and serum limitation. Cell Mol Neurobiol. 2008; 28:1067–1078.11. Silverstein JM, Musikantow D, Puente EC, Daphna-Iken D, Bree AJ, Fisher SJ. Pharmacologic amelioration of severe hypoglycemia-induced neuronal damage. Neurosci Lett. 2011; 492:23–28.12. Yousefzade G, Nakhaee A. Insulin-induced hypoglycemia and stress oxidative state in healthy people. Acta Diabetol. 2012; 49:Suppl 1. S81–S85.13. Wang J, Alexanian A, Ying R, Kizhakekuttu TJ, Dharmashankar K, Vasquez-Vivar J, Gutterman DD, Widlansky ME. Acute exposure to low glucose rapidly induces endothelial dysfunction and mitochondrial oxidative stress: role for AMP kinase. Arterioscler Thromb Vasc Biol. 2012; 32:712–720.14. Languren G, Montiel T, Julio-Amilpas A, Massieu L. Neuronal damage and cognitive impairment associated with hypoglycemia: an integrated view. Neurochem Int. 2013; 63:331–343.15. Fan L, Dang X, Shi Z, Zhang C, Wang K. Hydroxysafflor yellow A protects PC12 cells against the apoptosis induced by oxygen and glucose deprivation. Cell Mol Neurobiol. 2011; 31:1187–1194.16. Dong H, Mao S, Wei J, Liu B, Zhang Z, Zhang Q, Yan M. Tanshinone IIA protects PC12 cells from beta-amyloid(25-35)-induced apoptosis via PI3K/Akt signaling pathway. Mol Biol Rep. 2012; 39:6495–6503.17. Mao W, Iwai C, Keng PC, Vulapalli R, Liang CS. Norepinephrine-induced oxidative stress causes PC-12 cell apoptosis by both endoplasmic reticulum stress and mitochondrial intrinsic pathway: inhibition of phosphatidylinositol 3-kinase survival pathway. Am J Physiol Cell Physiol. 2006; 290:C1373–C1384.18. Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007; 7:847–859.19. Ho KK, Myatt SS, Lam EW. Many forks in the path: cycling with FoxO. Oncogene. 2008; 27:2300–2311.20. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999; 96:857–868.21. Kim JH, Choi JS, Lee BH. PI3K/Akt and MAPK pathways evoke activation of FoxO transcription factor to undergo neuronal apoptosis in brain of the silkworm Bombyx mori (Lepidoptera: Bombycidae). Cell Mol Biol (Noisy-le-grand). 2012; Suppl.58. OL1780–OL1785.22. Harris RJ, Wieloch T, Symon L, Siesjo BK. Cerebral extracellular calcium activity in severe hypoglycemia: relation to extracellular potassium and energy state. J Cereb Blood Flow Metab. 1984; 4:187–193.23. Sandberg M, Butcher SP, Hagberg H. Extracellular overflow of neuroactive amino acids during severe insulin-induced hypoglycemia: in vivo dialysis of the rat hippocampus. J Neurochem. 1986; 47:178–184.24. Ferrand-Drake M, Friberg H, Wieloch T. Mitochondrial permeability transition induced DNA-fragmentation in the rat hippocampus following hypoglycemia. Neuroscience. 1999; 90:1325–1338.25. McGowan JE, Chen L, Gao D, Trush M, Wei C. Increased mitochondrial reactive oxygen species production in newborn brain during hypoglycemia. Neurosci Lett. 2006; 399:111–114.26. Paramo B, Hernandez-Fonseca K, Estrada-Sanchez AM, Jimenez N, Hernandez-Cruz A, Massieu L. Pathways involved in the generation of reactive oxygen and nitrogen species during glucose deprivation and its role on the death of cultured hippocampal neurons. Neuroscience. 2010; 167:1057–1069.27. Mark RJ, Pang Z, Geddes JW, Uchida K, Mattson MP. Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J Neurosci. 1997; 17:1046–1054.28. Keller JN, Pang Z, Geddes JW, Begley JG, Germeyer A, Waeg G, Mattson MP. Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal. J Neurochem. 1997; 69:273–284.29. Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007; 117:910–918.30. Kirino T, Sano K. Selective vulnerability in the gerbil hippocampus following transient ischemia. Acta Neuropathol. 1984; 62:201–208.31. Lin HC, Narasimhan P, Liu SY, Chan PH, Lai IR. Postconditioning mitigates cell death following oxygen and glucose deprivation in PC12 cells and forebrain reperfusion injury in rats. J Neurosci Res. 2015; 93:140–148.32. Nakamura T, Minamisawa H, Katayama Y, Ueda M, Terashi A, Nakamura K, Kudo Y. Increased intracellular Ca2+ concentration in the hippocampal CA1 area during global ischemia and reperfusion in the rat: a possible cause of delayed neuronal death. Neuroscience. 1999; 88:57–67.33. Frantseva MV, Carlen PL, Perez Velazquez JL. Dynamics of intracellular calcium and free radical production during ischemia in pyramidal neurons. Free Radic Biol Med. 2001; 31:1216–1227.34. Shafaei-Bajestani N, Emami SA, Asili J, Tayarani-Najaran Z. Anti-apoptotic effect of taxodione on serum/glucose deprivation-induced PC12 cells death. Cell Mol Neurobiol. 2014; 34:1103–1109.35. Mousavi SH, Tayarani-Najaran Z, Asghari M, Sadeghnia HR. Protective effect of Nigella sativa extract and thymoquinone on serum/glucose deprivation-induced PC12 cells death. Cell Mol Neurobiol. 2010; 30:591–598.36. Zhao J, Bai Y, Zhang C, Zhang X, Zhang YX, Chen J, Xiong L, Shi M, Zhao G. Cinepazide maleate protects PC12 cells against oxygen-glucose deprivation-induced injury. Neurol Sci. 2014; 35:875–881.37. Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005; 24:7410–7425.38. Jacobs FM, van der Heide LP, Wijchers PJ, Burbach JP, Hoekman MF, Smidt MP. FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. J Biol Chem. 2003; 278:35959–35967.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The optimal model of reperfusion injury in vitro using H9c2 transformed cardiac myoblasts
- Inhibition of NF-kappaB Activation Increases Oxygen-Glucose Deprivation-Induced Cerebral Endothelial Cell Death
- Effects of pH, Buffer System and Lactate on the Simulated Ischemia-reperfusion Injury of H9c2 Cardiac Myocytes
- Nitric oxide prevents the bovine cerebral endothelial cell death induced by serum-deprivation
- The individual and combined neuroprotective effects of propofol and ketamine on rat mixed cortical cultures exposed to oxygen-glucose deprivation-reperfusion injury