Tuberc Respir Dis.
2012 Mar;72(3):275-283.
Impaired Expression of MAPK Is Associated with the Downregulation of TNF-alpha, IL-6, and IL-10 in Mycobacterium abscessus Lung Disease
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. wjkoh@skku.edu
- 2Department of Microbiology and Research Institute for Medical Sciences, Infection Signaling Network Research Center, Chungnam National University School of Medicine, Daejeon, Korea.
Abstract
- BACKGROUND
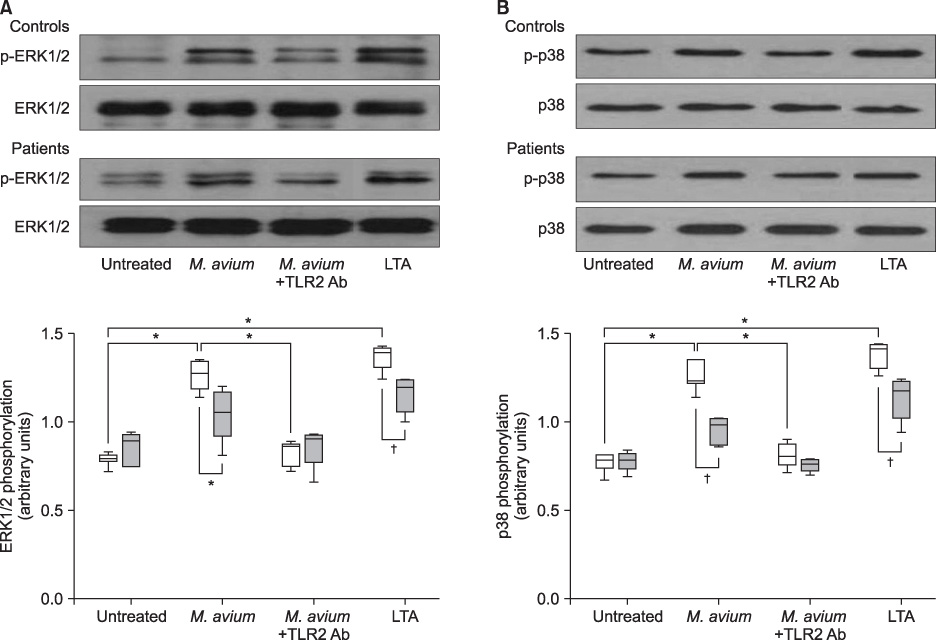
Healthy individuals who develop nontuberculous mycobacteria (NTM) lung disease are likely to have specific susceptibility factors which can lead to a NTM infection. The aim of the present study was to investigate the mechanism underlying innate immune responses, including the role of mitogen-activated protein kinase (MAPK), in Mycobacterium abscessus lung disease.
METHODS
Extracellular signal-regulated kinase (ERK1/2) and p38 MAPK expression in monocytes from peripheral blood mononuclear cells were measured by Western blot analysis after stimulation by Mycobacterium avium in five patients with M. abscessus lung disease and seven healthy controls. A M. avium-induced cytokine assay was performed after inhibition of ERK1/2 and p38 MAPK pathways.
RESULTS
Mycobacterium avium induced p38 and ERK1/2 expression in monocytes from healthy controls and subsequently upregulated tumor necrosis factor (TNF)-alpha, interleukin (IL)-6, and IL-10 production. In monocytes from patients with M. abscessus lung disease, however, induction of p38 and ERK1/2 expression, and the production of TNF-alpha, IL-6, and IL-10 were significantly lower.
CONCLUSION
Decreased activity of MAPK and cytokine secretion in monocytes from patients with M. abscessus lung disease may provide an explanation regarding host susceptibility to these uncommon infections.
Keyword
MeSH Terms
-
Blotting, Western
Down-Regulation
Extracellular Signal-Regulated MAP Kinases
Humans
Immunity, Innate
Interleukin-10
Interleukin-6
Interleukins
Lung
Lung Diseases
Mitogen-Activated Protein Kinases
Monocytes
Mycobacterium
Mycobacterium avium
Nontuberculous Mycobacteria
p38 Mitogen-Activated Protein Kinases
Phosphotransferases
Protein Kinases
Tumor Necrosis Factor-alpha
Extracellular Signal-Regulated MAP Kinases
Interleukin-10
Interleukin-6
Interleukins
Mitogen-Activated Protein Kinases
Phosphotransferases
Protein Kinases
Tumor Necrosis Factor-alpha
p38 Mitogen-Activated Protein Kinases
Figure
Reference
-
1. Brown-Elliott BA, Wallace RJ Jr. Clinical and taxonomic status of pathogenic nonpigmented or late-pigmenting rapidly growing mycobacteria. Clin Microbiol Rev. 2002. 15:716–746.2. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007. 175:367–416.3. Jeon K, Kwon OJ, Lee NY, Kim BJ, Kook YH, Lee SH, et al. Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am J Respir Crit Care Med. 2009. 180:896–902.4. Koh WJ, Jeon K, Lee NY, Kim BJ, Kook YH, Lee SH, et al. Clinical significance of differentiation of Mycobacterium massiliense from Mycobacterium abscessus. Am J Respir Crit Care Med. 2011. 183:405–410.5. Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, Daley CL. Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis. 2011. 52:565–571.6. Lyu J, Jang HJ, Song JW, Choi CM, Oh YM, Lee SD, et al. Outcomes in patients with Mycobacterium abscessus pulmonary disease treated with long-term injectable drugs. Respir Med. 2011. 105:781–787.7. Dailloux M, Abalain ML, Laurain C, Lebrun L, Loos-Ayav C, Lozniewski A, et al. Respiratory infections associated with nontuberculous mycobacteria in non-HIV patients. Eur Respir J. 2006. 28:1211–1215.8. Guide SV, Holland SM. Host susceptibility factors in mycobacterial infection: genetics and body morphotype. Infect Dis Clin North Am. 2002. 16:163–186.9. Koh WJ, Kwon OJ, Lee KS. Diagnosis and treatment of nontuberculous mycobacterial pulmonary diseases: a Korean perspective. J Korean Med Sci. 2005. 20:913–925.10. Diagnosis and treatment of disease caused by nontuberculous mycobacteria. This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association. Am J Respir Crit Care Med. 1997. 156(2 Pt 2):S1–S25.11. Ryu YJ, Kim EJ, Koh WJ, Kim H, Kwon OJ, Chang JH. Toll-like receptor 2 polymorphisms and nontuberculous mycobacterial lung diseases. Clin Vaccine Immunol. 2006. 13:818–819.12. Sampaio EP, Elloumi HZ, Zelazny A, Ding L, Paulson ML, Sher A, et al. Mycobacterium abscessus and M. avium trigger Toll-like receptor 2 and distinct cytokine response in human cells. Am J Respir Cell Mol Biol. 2008. 39:431–439.13. Shin DM, Yang CS, Yuk JM, Lee JY, Kim KH, Shin SJ, et al. Mycobacterium abscessus activates the macrophage innate immune response via a physical and functional interaction between TLR2 and dectin-1. Cell Microbiol. 2008. 10:1608–1621.14. Gomes MS, Flórido M, Cordeiro JV, Teixeira CM, Takeuchi O, Akira S, et al. Limited role of the Toll-like receptor-2 in resistance to Mycobacterium avium. Immunology. 2004. 111:179–185.15. Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001. 410:37–40.16. Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007. 7:179–190.17. Roach SK, Schorey JS. Differential regulation of the mitogen-activated protein kinases by pathogenic and nonpathogenic mycobacteria. Infect Immun. 2002. 70:3040–3052.18. Souza CD, Evanson OA, Weiss DJ. Role of the mitogen-activated protein kinase pathway in the differential response of bovine monocytes to Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium. Microbes Infect. 2007. 9:1545–1552.19. Tse HM, Josephy SI, Chan ED, Fouts D, Cooper AM. Activation of the mitogen-activated protein kinase signaling pathway is instrumental in determining the ability of Mycobacterium avium to grow in murine macrophages. J Immunol. 2002. 168:825–833.20. Shiratsuchi H, Ellner JJ, Basson MD. Extracellular-regulated kinase activation regulates replication of Mycobacterium avium intracellularly in primary human monocytes. Cell Tissue Res. 2008. 332:237–244.21. Bhattacharyya A, Pathak S, Kundu M, Basu J. Mitogen-activated protein kinases regulate Mycobacterium avium-induced tumor necrosis factor-alpha release from macrophages. FEMS Immunol Med Microbiol. 2002. 34:73–80.22. Klug K, Ehlers S, Uhlig S, Reiling N. Mitogen-activated protein kinases p38 and ERK1/2 regulated control of Mycobacterium avium replication in primary murine macrophages is independent of tumor necrosis factor-alpha and interleukin-10. Innate Immun. 2011. 17:470–485.23. Koh WJ, Lee KS, Kwon OJ, Jeong YJ, Kwak SH, Kim TS. Bilateral bronchiectasis and bronchiolitis at thin-section CT: diagnostic implications in nontuberculous mycobacterial pulmonary infection. Radiology. 2005. 235:282–288.24. Koh WJ, Kwon OJ, Jeon K, Kim TS, Lee KS, Park YK, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest. 2006. 129:341–348.25. Vankayalapati R, Wizel B, Samten B, Griffith DE, Shams H, Galland MR, et al. Cytokine profiles in immunocompetent persons infected with Mycobacterium avium complex. J Infect Dis. 2001. 183:478–484.26. Ryu YJ, Kim EJ, Lee SH, Kim SY, Suh GY, Chung MP, et al. Impaired expression of Toll-like receptor 2 in nontuberculous mycobacterial lung disease. Eur Respir J. 2007. 30:736–742.27. Kwon YS, Kim EJ, Lee SH, Suh GY, Chung MP, Kim H, et al. Decreased cytokine production in patients with nontuberculous mycobacterial lung disease. Lung. 2007. 185:337–341.28. Greinert U, Schlaak M, Rüsch-Gerdes S, Flad HD, Ernst M. Low in vitro production of interferon-gamma and tumor necrosis factor-alpha in HIV-seronegative patients with pulmonary disease caused by nontuberculous mycobacteria. J Clin Immunol. 2000. 20:445–452.29. Feng CG, Scanga CA, Collazo-Custodio CM, Cheever AW, Hieny S, Caspar P, et al. Mice lacking myeloid differentiation factor 88 display profound defects in host resistance and immune responses to Mycobacterium avium infection not exhibited by Toll-like receptor 2 (TLR2)- and TLR4-deficient animals. J Immunol. 2003. 171:4758–4764.30. Stenger S. Immunological control of tuberculosis: role of tumour necrosis factor and more. Ann Rheum Dis. 2005. 64:Suppl 4. iv24–iv28.31. Wallis RS, Ellner JJ. Cytokines and tuberculosis. J Leukoc Biol. 1994. 55:676–681.32. Shiratsuchi H, Johnson JL, Ellner JJ. Bidirectional effects of cytokines on the growth of Mycobacterium avium within human monocytes. J Immunol. 1991. 146:3165–3170.33. Ladel CH, Blum C, Dreher A, Reifenberg K, Kopf M, Kaufmann SH. Lethal tuberculosis in interleukin-6-deficient mutant mice. Infect Immun. 1997. 65:4843–4849.34. Yadav M, Roach SK, Schorey JS. Increased mitogen-activated protein kinase activity and TNF-alpha production associated with Mycobacterium smegmatis- but not Mycobacterium avium-infected macrophages requires prolonged stimulation of the calmodulin/calmodulin kinase and cyclic AMP/protein kinase A pathways. J Immunol. 2004. 172:5588–5597.35. Hasan Z, Shah BH, Mahmood A, Young DB, Hussain R. The effect of mycobacterial virulence and viability on MAP kinase signalling and TNF alpha production by human monocytes. Tuberculosis (Edinb). 2003. 83:299–309.36. Reiling N, Blumenthal A, Flad HD, Ernst M, Ehlers S. Mycobacteria-induced TNF-alpha and IL-10 formation by human macrophages is differentially regulated at the level of mitogen-activated protein kinase activity. J Immunol. 2001. 167:3339–3345.37. Sable SB, Goyal D, Verma I, Behera D, Khuller GK. Lung and blood mononuclear cell responses of tuberculosis patients to mycobacterial proteins. Eur Respir J. 2007. 29:337–346.38. Schwander SK, Torres M, Carranza CC, Escobedo D, Tary-Lehmann M, Anderson P, et al. Pulmonary mononuclear cell responses to antigens of Mycobacterium tuberculosis in healthy household contacts of patients with active tuberculosis and healthy controls from the community. J Immunol. 2000. 165:1479–1485.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concentration of IL-1B, IL-6, IL-8, TNF-a in Cerebrospinal Fluid of Patients with Meningitis and Control
- Role of Mitogen-Activated Protein Kinase Pathways in the Production of TNF-alpha, IL-10, and MCP-1 by Mycobacterium tuberculosis H37Rv-Infected Human Blood Monocytes
- Differential Roles of Toll-like Receptor (TLR) 2 and 4 between PPD- and 38-kDa-induced Proinflammatory Cytokine Productions in Human Monocytes
- Regulation of TNF - alpha Gene Expression in Human Fetal Astrocytes
- Blockade of p38 Mitogen-activated Protein Kinase Pathway Inhibits Interleukin-6 Release and Expression in Primary Neonatal Cardiomyocytes