Tuberc Respir Dis.
2010 Nov;69(5):337-347.
The Role of Transglutaminase-2 in Fibroproliferation after Lipopolysaccharide-induced Acute Lung Injury
- Affiliations
-
- 1Division of Pulmonary, Sleep and Critical Care Medicine, Department of Internal Medicine, Korea University Ansan Hospital, Ansan, Korea. chepraxis@korea.ac.kr
Abstract
- BACKGROUND
Transglutaminase-2 (TG-2) has been reported to play an important role in the process of fibrosis. However, TG-2 studies on fibroproliferation of acute lung injury (ALI) are absent. The purpose of this study was to investigate the role of TG-2 in the fibroproliferation of lipopolysaccharide (LPS)-induced ALI.
METHODS
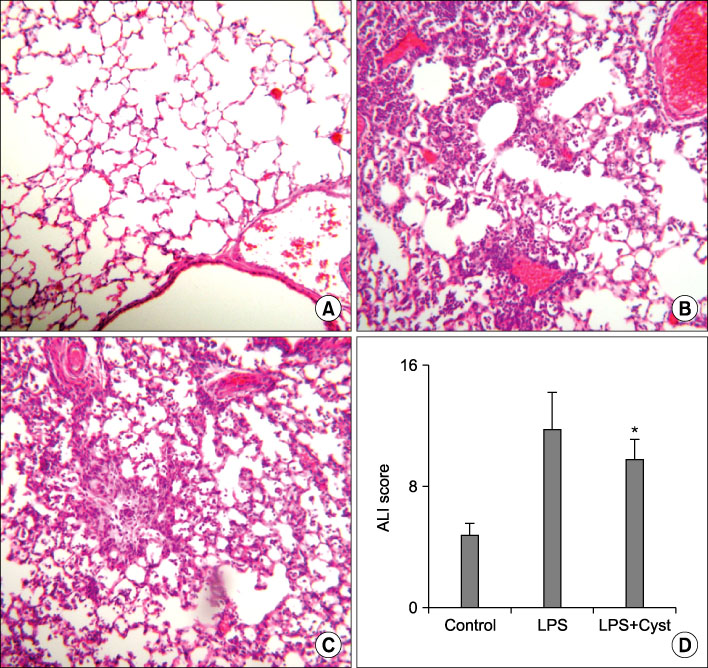
The male C57BL/6 mice of 5 weeks age were divided into 3 groups; control group (n=30) in which 50 microL of saline was given intratracheally (IT), LPS group (n=30) in which LPS 0.5 mg/kg/50 microL of saline was given IT, and LPS+Cyst group treated with intraperitoneal 200 mg/kg of cystamine, competitive inhibitor of TG-2, after induction of ALI by LPS. TG-2 activity and nuclear factor (NF)-kappaB were measured in lung tissue homogenate. Tumor necrosis factor (TNF)-alpha, interleukin (IL)-1beta, IL-6, myeloperoxidase (MPO), and transforming growth factor (TGF)-beta1 were measured using bronchoalveolar lavage fluids. Histopathologic ALI score and Mallory's phosphotunistic acid hematoxylin (PTAH) for collagen and fibronectin deposition were performed.
RESULTS
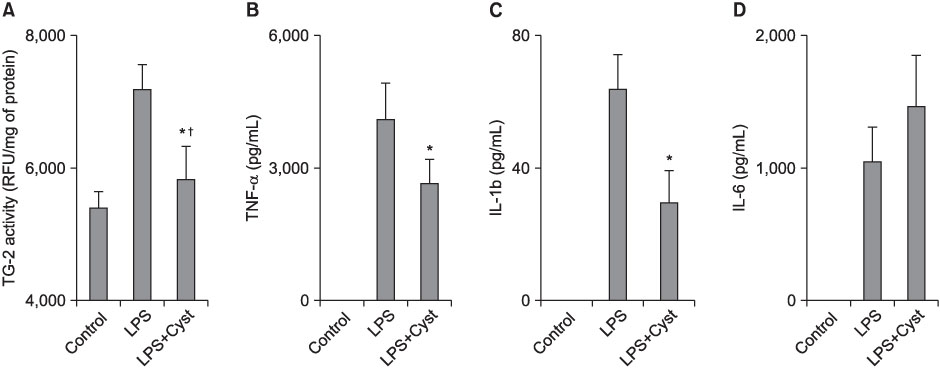
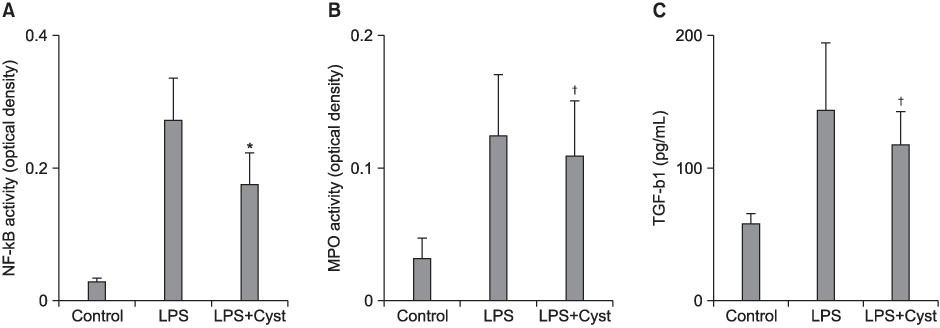
The TG-2 activities in the LPS group were significantly higher than the control and LPS+Cyst groups (p<0.05). The TNF-alpha and IL-1beta concentrations and NF-kappaB activity were lower in the LPS+Cyst group than the LPS group (p<0.05). The LPS+Cyst group showed lower MPO, ALI score, TGF-beta1 concentration, and Mallory's PTAH stain than the LPS group, but the differences were not significant (p>0.05).
CONCLUSION
Inhibition of TG-2 activity in the LPS-induced ALI prevented early inflammatory parameters, but had limited effects on late ALI and fibroproliferative parameters.
Keyword
MeSH Terms
-
Acute Lung Injury
Animals
Bronchoalveolar Lavage Fluid
Collagen
Cystamine
Fibronectins
Fibrosis
Hematoxylin
Humans
Inflammation
Interleukin-6
Interleukins
Lipopolysaccharides
Lung
Male
Mice
NF-kappa B
Peroxidase
Transforming Growth Factor beta1
Transforming Growth Factors
Tumor Necrosis Factor-alpha
Collagen
Cystamine
Fibronectins
Hematoxylin
Interleukin-6
Interleukins
Lipopolysaccharides
NF-kappa B
Peroxidase
Transforming Growth Factor beta1
Transforming Growth Factors
Tumor Necrosis Factor-alpha
Figure
Reference
-
1. Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005. 353:1685–1693.2. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000. 342:1334–1349.3. Desai SR, Wells AU, Rubens MB, Evans TW, Hansell DM. Acute respiratory distress syndrome: CT abnormalities at long-term follow-up. Radiology. 1999. 210:29–35.4. McHugh LG, Milberg JA, Whitcomb ME, Schoene RB, Maunder RJ, Hudson LD. Recovery of function in survivors of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1994. 150:90–94.5. Davidson TA, Caldwell ES, Curtis JR, Hudson LD, Steinberg KP. Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA. 1999. 281:354–360.6. Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003. 348:683–693.7. Armstrong L, Thickett DR, Mansell JP, Ionescu M, Hoyle E, Billinghurst RC, et al. Changes in collagen turnover in early acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999. 160:1910–1915.8. Marshall RP, Bellingan G, Webb S, Puddicombe A, Goldsack N, McAnulty RJ, et al. Fibroproliferation occurs early in the acute respiratory distress syndrome and impacts on outcome. Am J Respir Crit Care Med. 2000. 162:1783–1788.9. Griffin M, Casadio R, Bergamini CM. Transglutaminases: nature's biological glues. Biochem J. 2002. 368:377–396.10. Folk JE, Finlayson JS. The epsilon-(gamma-glutamyl)lysine crosslink and the catalytic role of transglutaminases. Adv Protein Chem. 1977. 31:1–133.11. Kim SY, Jeitner TM, Steinert PM. Transglutaminases in disease. Neurochem Int. 2002. 40:85–103.12. Aeschlimann D, Thomazy V. Protein crosslinking in assembly and remodelling of extracellular matrices: the role of transglutaminases. Connect Tissue Res. 2000. 41:1–27.13. Johnson TS, Skill NJ, El Nahas AM, Oldroyd SD, Thomas GL, Douthwaite JA, et al. Transglutaminase transcription and antigen translocation in experimental renal scarring. J Am Soc Nephrol. 1999. 10:2146–2157.14. Shweke N, Boulos N, Jouanneau C, Vandermeersch S, Melino G, Dussaule JC, et al. Tissue transglutaminase contributes to interstitial renal fibrosis by favoring accumulation of fibrillar collagen through TGF-beta activation and cell infiltration. Am J Pathol. 2008. 173:631–642.15. Mirza A, Liu SL, Frizell E, Zhu J, Maddukuri S, Martinez J, et al. A role for tissue transglutaminase in hepatic injury and fibrogenesis, and its regulation by NF-kappaB. Am J Physiol. 1997. 272:G281–G288.16. Johnson TS, Fisher M, Haylor JL, Hau Z, Skill NJ, Jones R, et al. Transglutaminase inhibition reduces fibrosis and preserves function in experimental chronic kidney disease. J Am Soc Nephrol. 2007. 18:3078–3088.17. Qiu JF, Zhang ZQ, Chen W, Wu ZY. Cystamine ameliorates liver fibrosis induced by carbon tetrachloride via inhibition of tissue transglutaminase. World J Gastroenterol. 2007. 13:4328–4332.18. Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003. 116:217–224.19. Shin DM, Jeon JH, Kim CW, Cho SY, Lee HJ, Jang GY, et al. TGFbeta mediates activation of transglutaminase 2 in response to oxidative stress that leads to protein aggregation. FASEB J. 2008. 22:2498–2507.20. Verderio EA, Johnson T, Griffin M. Tissue transglutaminase in normal and abnormal wound healing: review article. Amino Acids. 2004. 26:387–404.21. Kim JH, Suk MH, Yoon DW, Kim HY, Jung KH, Kang EH, et al. Inflammatory and transcriptional roles of poly (ADP-ribose) polymerase in ventilator-induced lung injury. Crit Care. 2008. 12:R108.22. Rojas M, Woods CR, Mora AL, Xu J, Brigham KL. Endotoxin-induced lung injury in mice: structural, functional, and biochemical responses. Am J Physiol Lung Cell Mol Physiol. 2005. 288:L333–L341.23. Jeitner TM, Delikatny EJ, Ahlqvist J, Capper H, Cooper AJ. Mechanism for the inhibition of transglutaminase 2 by cystamine. Biochem Pharmacol. 2005. 69:961–970.24. Tomashefski JF Jr. Pulmonary pathology of acute respiratory distress syndrome. Clin Chest Med. 2000. 21:435–466.25. Chesnutt AN, Matthay MA, Tibayan FA, Clark JG. Early detection of type III procollagen peptide in acute lung injury. Pathogenetic and prognostic significance. Am J Respir Crit Care Med. 1997. 156:840–845.26. Steinberg KP, Hudson LD. Acute lung injury and acute respiratory distress syndrome: the clinical syndrome. Clin Chest Med. 2000. 21:401–417.27. Martin C, Papazian L, Payan MJ, Saux P, Gouin F. Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome: a study in mechanically ventilated patients. Chest. 1995. 107:196–200.28. Dhainaut JF, Charpentier J, Chiche JD. Transforming growth factor-beta: a mediator of cell regulation in acute respiratory distress syndrome. Crit Care Med. 2003. 31:4 Suppl. S258–S264.29. Grenard P, Bates MK, Aeschlimann D. Evolution of transglutaminase genes: identification of a transglutaminase gene cluster on human chromosome 15q15. Structure of the gene encoding transglutaminase X and a novel gene family member, transglutaminase Z. J Biol Chem. 2001. 276:33066–33078.30. Park KC, Chung KC, Kim YS, Lee J, Joh TH, Kim SY. Transglutaminase 2 induces nitric oxide synthesis in BV-2 microglia. Biochem Biophys Res Commun. 2004. 323:1055–1062.31. Lee ZW, Kwon SM, Kim SW, Yi SJ, Kim YM, Ha KS. Activation of in situ tissue transglutaminase by intracellular reactive oxygen species. Biochem Biophys Res Commun. 2003. 305:633–640.32. Shin DM, Jeon JH, Kim CW, Cho SY, Kwon JC, Lee HJ, et al. Cell type-specific activation of intracellular transglutaminase 2 by oxidative stress or ultraviolet irradiation: implications of transglutaminase 2 in age-related cataractogenesis. J Biol Chem. 2004. 279:15032–15039.33. Lorand L, Weissmann LB, Epel DL, Bruner-Lorand J. Role of the intrinsic transglutaminase in the Ca2+-mediated crosslinking of erythrocyte proteins. Proc Natl Acad Sci U S A. 1976. 73:4479–4481.34. Scott KF, Meyskens FL Jr, Russell DH. Retinoids increase transglutaminase activity and inhibit ornithine decarboxylase activity in Chinese hamster ovary cells and in melanoma cells stimulated to differentiate. Proc Natl Acad Sci U S A. 1982. 79:4093–4097.35. Esposito C, Paparo F, Caputo I, Porta R, Salvati VM, Mazzarella G, et al. Expression and enzymatic activity of small intestinal tissue transglutaminase in celiac disease. Am J Gastroenterol. 2003. 98:1813–1820.36. Kuncio GS, Tsyganskaya M, Zhu J, Liu SL, Nagy L, Thomazy V, et al. TNF-alpha modulates expression of the tissue transglutaminase gene in liver cells. Am J Physiol. 1998. 274:G240–G245.37. Roberts ES, Zandonatti MA, Watry DD, Madden LJ, Henriksen SJ, Taffe MA, et al. Induction of pathogenic sets of genes in macrophages and neurons in Neuro-AIDS. Am J Pathol. 2003. 162:2041–2057.38. Lee J, Kim YS, Choi DH, Bang MS, Han TR, Joh TH, et al. Transglutaminase 2 induces nuclear factor-kappaB activation via a novel pathway in BV-2 microglia. J Biol Chem. 2004. 279:53725–53735.39. Griffin M, Smith LL, Wynne J. Changes in transglutaminase activity in an experimental model of pulmonary fibrosis induced by paraquat. Br J Exp Pathol. 1979. 60:653–661.40. Suh GY, Ham HS, Lee SH, Choi JC, Koh WJ, Kim SY, et al. A Peptide with anti-transglutaminase activity decreases lipopolysaccharide-induced lung inflammation in mice. Exp Lung Res. 2006. 32:43–53.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Transglutaminase 2 Inhibition on Ventilator-Induced Lung Injury
- Diosmetin Alleviates Lipopolysaccharide-Induced Acute Lung Injury through Activating the Nrf2 Pathway and Inhibiting the NLRP3 Inflammasome
- Role of the PLA2-Activated Neutrophilic Oxidative Stress in Oleic Acid-Induced Acute Lung Injury
- Phospholipase A2 Contributes to Hemorrhage-induced Acute Lung Injury Through Neutrophilic Respiratory Burst
- The role of pulmonary capillary pressure in the oxygen free radical- induced acute lung injury