Tuberc Respir Dis.
2006 Mar;60(3):304-313.
The Role of Heme Oxygenase-1 in Lung Cancer Cells
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine Wonkwang University, Iksan, Korea. kshryj@wonkwang.ac.kr
Abstract
-
BACKGROUND: Heme oxygenase-1 (HO-1) is an inducible enzyme that catalyzes the oxidative degradation of heme to form biliverdin, carbon monoxide (CO), and free iron. The current evidence has indicated a critical role of HO-1 in cytoprotection and also in other, more diverse biological functions. It is known that the high expression of HO-1 occurs in various tumors, and that HO-1 has an important role in rapid tumor growth because of its antioxidative and antiapoptotic effects. Therefore, the role of HO-1 was analyzed in human lung cancer cell lines, and especially in the A549 cell line.
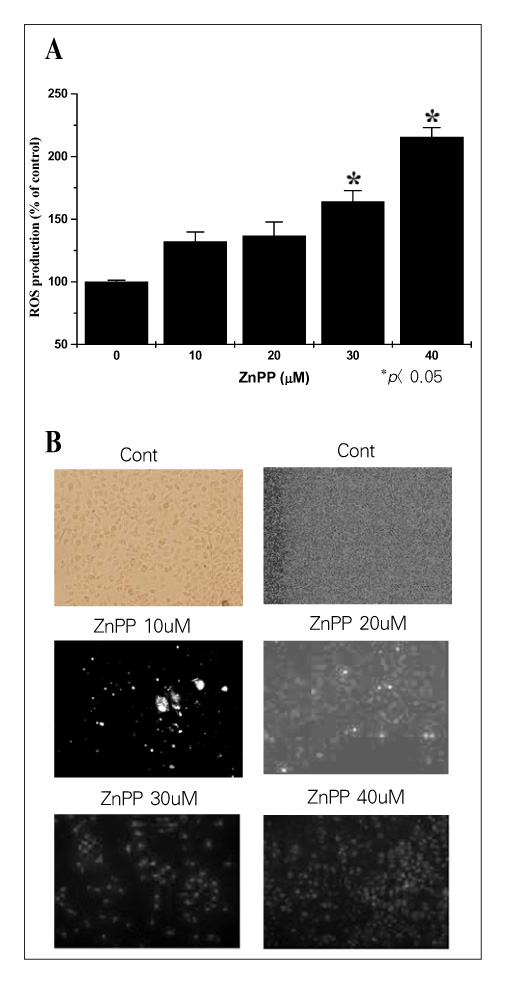
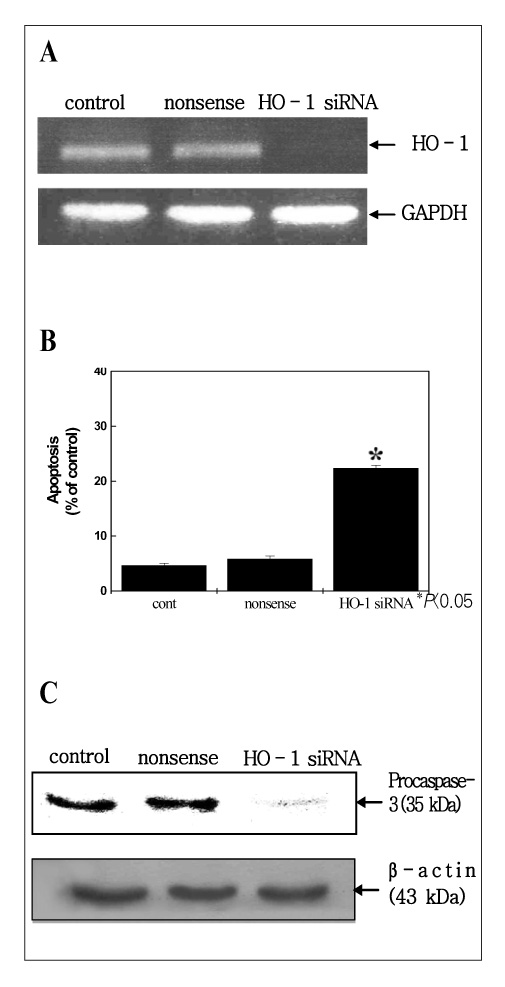
MATERIAL AND METHODS: Human lung cancer cell lines, i.e., A549, NCI-H23, NCI-H157 and NCI-H460, were used for this study. The expression of HO-1 in the untreated state was defined by Western blotting. ZnPP, which is the specific HO inhibitor we used, and the viability of cells were tested for by conducting MTT assaysy. The HO enzymatic activity, as determined via the bilirubin level, was also indirectly measured. Moreover, the generation of intracellular hydrogen peroxide (H2O2) was monitored fluorimetrically with using a scopoletin-horse radish peroxidase (HRP) assay and 2',7'-dichlorofluorescein diacetate (DCFH-DA). We have also transfected small HO-1 interfering RNA (siRNA) into A549 cells, and the apoptotic effects were evaluated by flow cytometric analysis and Western blotting.
RESULTS
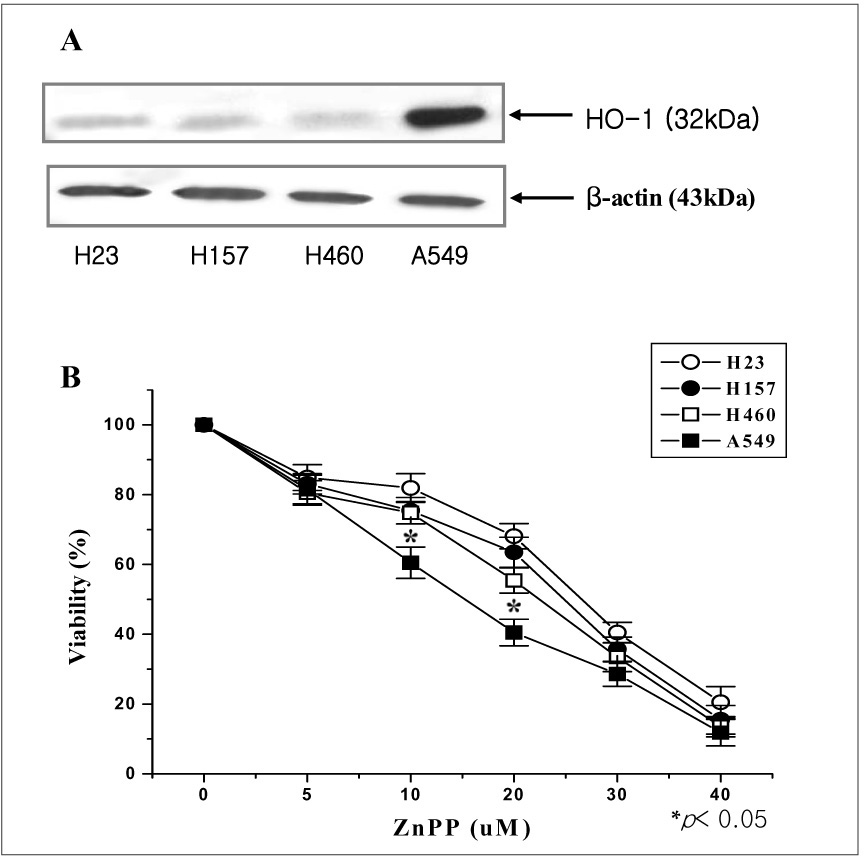
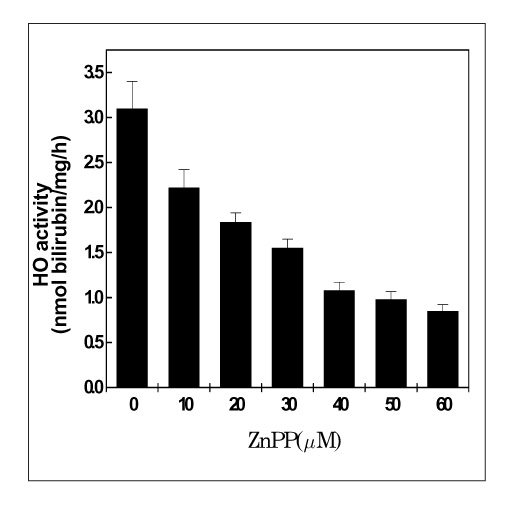
The A549 cells had a greater expression of HO-1 than the other cell lines, whereas ZnPP significantly decreased the viability of the A549 cells more than the viability of the other lung cancer cells in a dose-dependant fashion. Consistent with the viability, the HO enzymatic activity also was decreased. Moreover, intracellular H2O2 generation via ZnPP was induced in a dose-dependent manner. Apoptotic events were, then induced in the HO-1 siRNA transfected A549 cells.
CONCLUSION
HO-1 provides new important insights into the possible molecular mechanism of the antitumor therapy in lung cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Bae JM, Won YJ, Jung KW, Suh KA, Yun YH, Shin MH, et al. SurvivalKorean cancer patients diagnosed in 1995. Cancer Res Treat. 2002. 34:319–325.2. Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002. 346:92–98.3. Giaccone G, Herbst RS, Manegold C, Scagliotti G, Resell R, Miller V, et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-sm all-cell lung cancer: a phase III trial-INTACT 1. J Clin Oncol. 2004. 22:777–784.4. Sandler AB, Johnson DH, Herbst RS. Anti-vascular endothelial growth factor monoclonals in non-small cell lung cancer. Clin Cancer Res. 2004. 10:4258S–4262S.5. Tenhunen R, Marver HS, Schmid R. The enzymatic catabolism of hemoglobin: stimulation of microsomal heme oxygenase by hemin. J Lab Clin Med. 1970. 75:410–421.6. Baranano DE, Rao M, Ferris CD, Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci U S A. 2002. 99:16093–16098.7. McCoubrey WK Jr, Huang TJ, Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem. 1997. 247:725–732.8. Motterlini R, Foresti R, Bassi R, Calabrese V, Clark JE, Green CJ. Endothelial heme oxygenase-1 induction by hypoxia: modulation by inducible nitric-oxide synthase and S-nitrosothiols. J Biol Chem. 2000. 275:13613–13620.9. Doi K, Akaike T, Fujii S, Tanaka S, Ikebe N, Beppu T, et al. Induction of haem oxygenase-1 nitric oxide and ischaemia in experimental solid tumours and implications for tumour growth. Br J Cancer. 1999. 80:1945–1954.10. Elbirt KK, Whitmarsh AJ, Davis RJ, Bonkovsky HL. Mechanism of sodium arsenite-mediated induction of heme oxygenase-1 in hepatoma cells: role of mitogen-activated protein kinases. J Biol Chem. 1998. 273:8922–8931.11. Eyssen-Hernandez R, Ladoux A, Frelin C. Differential regulation of cardiac heme oxygenase-1 and vascular endothelial growth factor mRNA expressions by hemin, heavy metals, heat shock and anoxia. FEBS Lett. 1996. 382:229–233.12. Keyse SM, Tyrrell RM. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989. 86:99–103.13. Lautier D, Luscher P, Tyrrell RM. Endogenous glutathione levels modulate both constitutive and UVA radiation/hydrogen peroxide inducible expression of the human heme oxygenase gene. Carcinogenesis. 1992. 13:227–232.14. Wagner M, Cadetg P, Ruf R, Mazzucchelli L, Ferrari P, Redaelli CA. Heme oxygenase-1 attenuates ischemia/ reperfusion-induced apoptosis and improves survival in rat renal allografts. Kidney Int. 2003. 63:1564–1573.15. Choi BM, Pae HO, Chung HT. Nitric oxide priming protects nitric oxide-mediated apoptosis via heme oxygenase-1 induction. Free Radic Biol Med. 2003. 34:1136–1145.16. Amon M, Menger MD, Vollmar B. Heme oxygenase and nitric oxide synthase mediate cooling-associated protection against TNF-alpha-induced microcirculatory dysfunction and apoptotic cell death. FASEB J. 2003. 17:175–185.17. Ke B, Shen XD, Zhai Y, Gao F, Busuttil RW, Volk HD, et al. Heme oxygenase 1 mediates the immunomodulatory and antiapoptotic effects of interleukin 13 gene therapy in vivo and in vitro. Hum Gene Ther. 2002. 13:1845–1857.18. Goodman AI, Choudhury M, da Silva JL, Schwartzman ML, Abraham NG. Overexpression of the heme oxygenase gene in renal cell carcinoma. Proc Soc Exp Biol Med. 1997. 214:54–61.19. Maines MD, Abrahamsson PA. Expression of heme oxygenase-1 (HSP32) in human prostate: normal, hyperplastic, and tumor tissue distribution. Urology. 1996. 47:727–733.20. Sahoo SK, Sawa T, Fang J, Tanaka S, Miyamoto Y, Akaike T, et al. Pegylated zinc protoporphyrin: a water-soluble heme oxygenase inhibitor with tumor-targeting capacity. Bioconjug Chem. 2002. 13:1031–1038.21. Pae HO, Oh GS, Choi BM, Chae SC, Kim YM, Chung KR, et al. Carbon monoxide produced by heme oxygenase-1 suppresses T cell proliferation via inhibition of IL-2 production. J Immunol. 2004. 172:4744–4751.22. Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, et al. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000. 6:422–428.23. Berberat PO, Katori M, Kaczmarek E, Anselmo D, Lassman C, Ke B, et al. Heavy chain ferritin acts as an antiapoptotic gene that protects livers from ischemia reperfusion injury. FASEB J. 2003. 17:1724–1726.24. Lee PJ, Alam J, Wiegand GW, Choi AM. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc Natl Acad Sci U S A. 1996. 93:10393–10398.25. Dennery PA, Sridhar KJ, Lee CS, Wong HE, Shokoohi V, Rodgers PA, et al. Heme oxygenase-mediated resistance to oxygen toxicity in hamster fibroblasts. J Biol Chem. 1997. 272:14937–14942.26. Otterbein L, Chin BY, Otterbein SL, Lowe VC, Fessler HE, Choi AM. Mechanism of hemoglobin-induced protection against endotoxemia in rats: a ferritin-independent pathway. Am J Physiol. 1997. 272:L268–L275.27. Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, et al. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999. 103:129–135.28. Clark JE, Green CJ, Motterlini R. Involvement of the heme oxygenase-carbon monoxide pathway in keratinocyte proliferation. Biochem Biophys Res Commun. 1997. 241:215–220.29. Deramaudt BM, Braunstein S, Remy P, Abraham NG. Gene transfer of human heme oxygenase into coronary endothelial cells potentially promotes angiogenesis. J Cell Biochem. 1998. 68:121–127.30. Tsuji MH, Yanagawa T, Iwasa S, Tabuchi K, Onizawa K, Bannai S, et al. Heme oxygenase-1 expression in oral squamous cell carcinoma as involved in lymph node metastasis. Cancer Lett. 1999. 138:53–59.31. Deininger MH, Meyermann R, Trautmann K, Duffner F, Grote EH, Wickboldt J, et al. Heme oxygenase (HO)-1 expressing macrophages/microglial cells accumulate during oligodendroglioma progression. Brain Res. 2000. 882:1–8.32. Torisu-Itakura H, Furue M, Kuwano M, Ono M. Co-expression of thymidine phosphorylase and heme oxygenase-1 in macrophages in human malignant vertical growth melanomas. Jpn J Cancer Res. 2000. 91:906–910.33. Fang J, Sawa T, Akaike T, Akuta T, Sahoo SK, Khaled G, et al. In vivo antitumor activity of pegylated zinc protoporphyrin: targeted inhibition of heme oxygenase in solid tumor. Cancer Res. 2003. 63:3567–3574.34. Fang J, Akaike T, Maeda H. Antiapoptotic role of heme oxygnease (HO) and the potential of HO as a target in anticancer treatment. Apoptosis. 2004. 9:27–35.35. Tanaka S, Akaike T, Fang J, Beppu T, Ogawa M, Tamura F, et al. Antiapoptotic effect of haem oxygenase-1 induced by nitric oxide in experimental solid tumor. Br J Cancer. 2003. 88:902–909.36. Dumont A, Hehner SP, Hofmann TG, Ueffing M, Droge W, Schmitz ML. Hydrogen peroxide-induced apoptosis is CD95-independent, requires the release of mitochondria-derived reactive oxygen species and the activation of NF-kappaB. Oncogene. 1999. 18:747–757.37. Slater AF, Stefan C, Nobel I, van den Dobbelsteen DJ, Orrenius S. Signalling mechanisms and oxidative stress in apoptosis. Toxicol Lett. 1995. 82/83:149–153.38. Vile GF, Tyrrell RM. Oxidative stress resulting from ultraviolet A irradiation of human skin fibroblasts leads to a heme oxygenase-dependent increase in ferritin. J Biol Chem. 1993. 268:14678–14681.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Heme Oxygenase-1 via Transcriptional Activation of Nrf2 Attenuates Apoptosis in Lung Cancer Cells
- Heme Oxygenase-1: Its Therapeutic Roles in Inflammatory Diseases
- Role of Carbon Monoxide in Neurovascular Repair Processing
- Ischemia/reperfusion Lung Injury Increases Serum Ferritin and Heme Oxygenase-1 in Rats
- The Effect of Inhibition of Heme Oxygenase-1 on Chemosensitivity of Cisplatin in Lung Cancer Cells