Korean J Physiol Pharmacol.
2009 Jun;13(3):181-187. 10.4196/kjpp.2009.13.3.181.
Ischemia/reperfusion Lung Injury Increases Serum Ferritin and Heme Oxygenase-1 in Rats
- Affiliations
-
- 1Department of Physiology, School of Medicine, Catholic University of Daegu, Daegu 705-718, Korea. yypark@cu.ac.kr
- KMID: 2071669
- DOI: http://doi.org/10.4196/kjpp.2009.13.3.181
Abstract
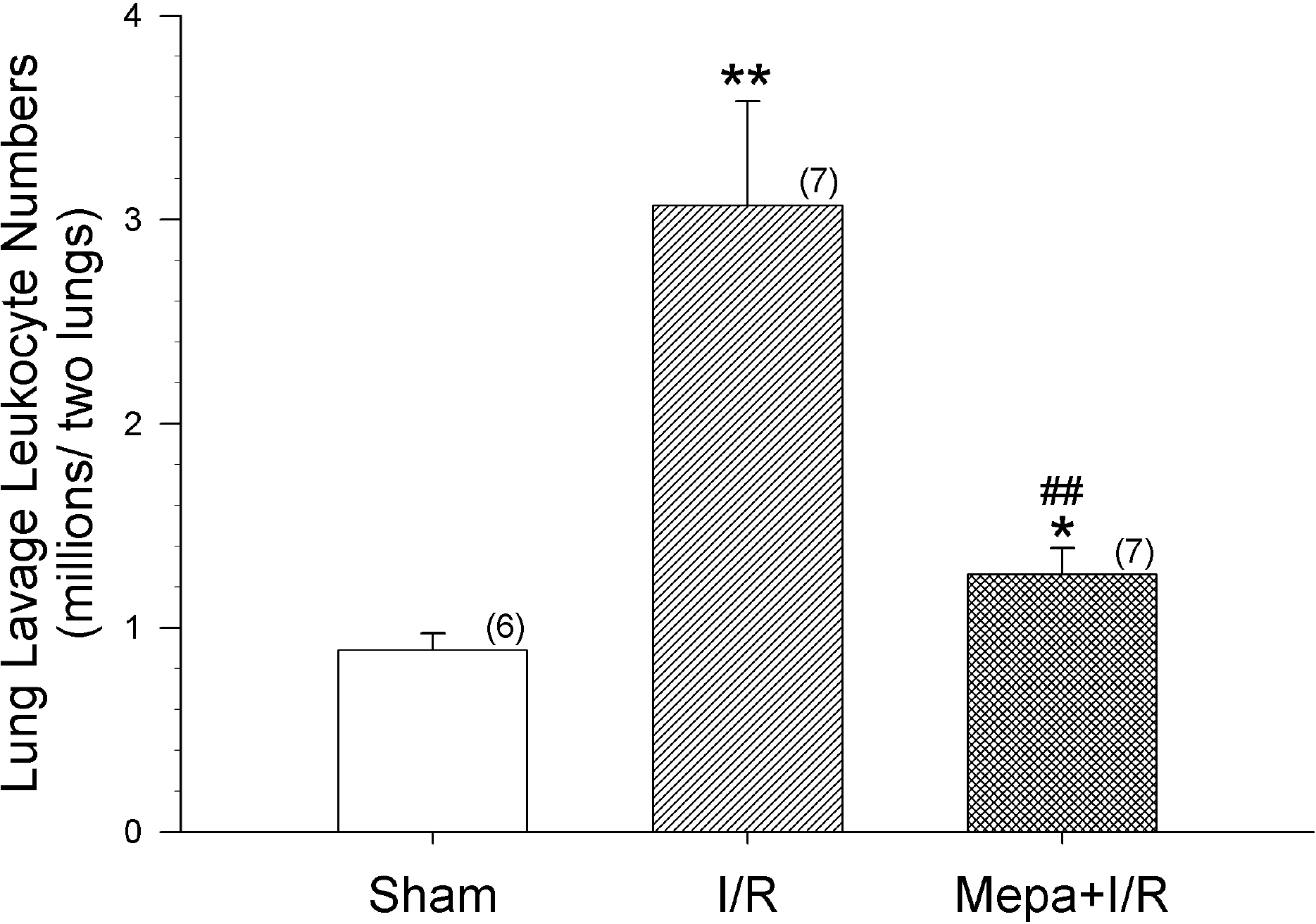
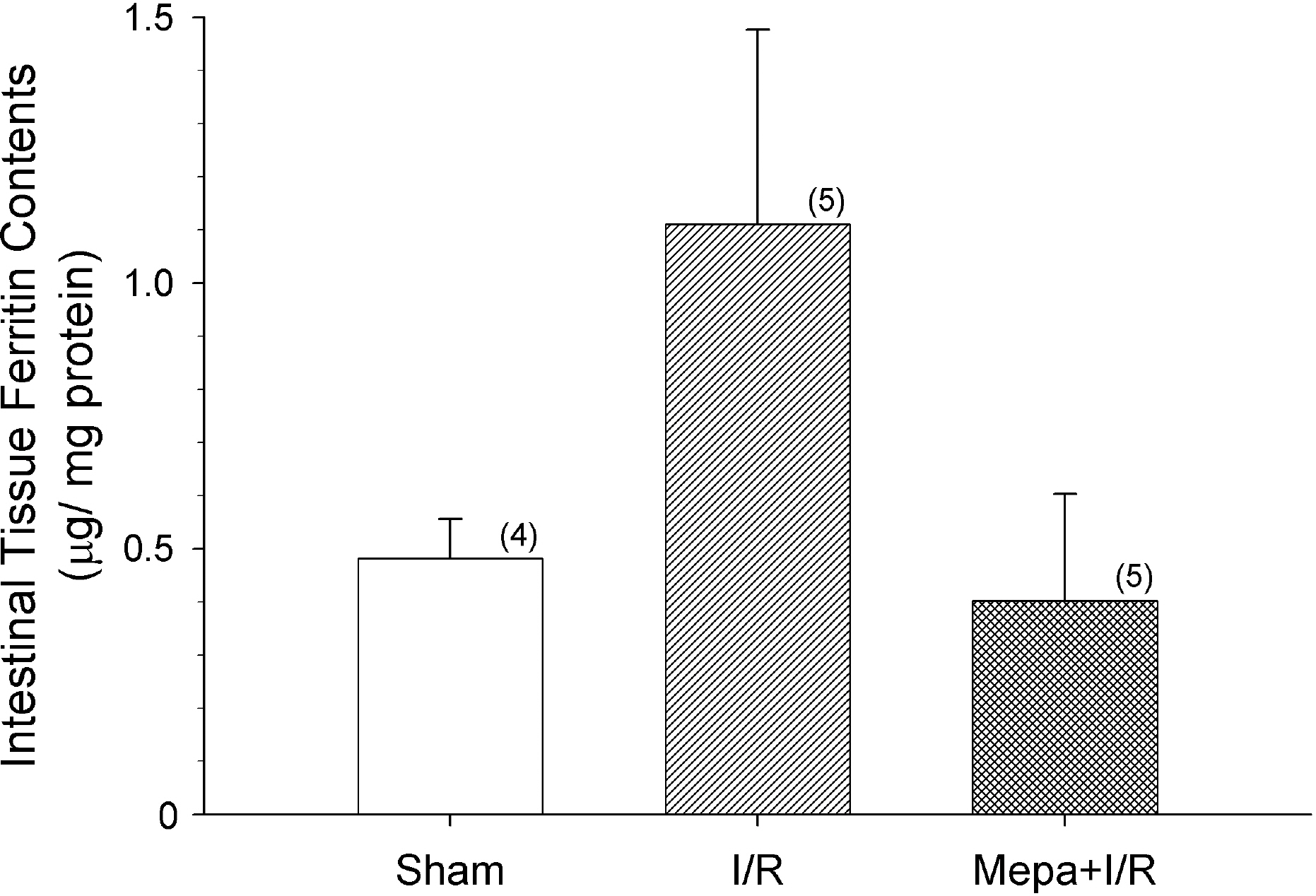
- Intestinal ischemia/reperfusion (I/R) is one of common causes of acute lung injury (ALI). Early and accurate diagnosis of patients who are like to develop serious acute respiratory distress syndrome (ARDS) would give a therapeutic advantage. Ferritin and heme oxygenase-1 (HO-1) are increased by oxidative stress and are potential candidates as a predictive biomarker of ARDS. However, the mechanisms responsible for the increases of ferritin and HO-1, and their relationship to ALI, are unclear. In order to elucidate the interactions between ferritin and HO-1, we studied the changes in ferritin and HO-1 levels in serum and bronchoalveolar lavage (BAL) fluid after intestinal I/R injury in rats. Leukocyte number and protein contents in BAL fluid were elevated following I/R, and the increases were attenuated by mepacrine pretreatment. Both serum ferritin and HO-1 concentrations were progressively elevated throughout the 3 h observation period. Mepacrine pretreatment attenuated the increase of serum and BAL fluid ferritin concentrations, but did not suppress the increase of serum HO-1. Moreover, BAL fluid HO-1 levels did not change after I/R or after mepacrine pretreated I/R compared with sham rats. Unlike ferritin, HO-1 levels are not exactly matched with the ALI. Therefore, there might be a different mechanism between the changes of ferritin and HO-1 in intestinal I/R-induced ALI model.
MeSH Terms
Figure
Reference
-
Amersi F., Buelow R., Kato H., Ke B., Coito AJ., Shen XD., Zhao D., Zaky J., Melinek J., Lassman CR., Kolls JK., Alam J., Ritter T., Volk HD., Farmer DG., Ghobrial RM., Busuttil RW., Kupiec-Weglinski JW. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Invest. 104:1631–1639. 1999.
ArticleAttuwaybi BO., Hassoun HT., Zou L., Kozar RA., Kone BC., Weisbrodt NW., Moore FA. Hypothermia protects against gut ischemia reperfusion-induced impaired intestinal transit by inducing heme oxygenase-1. J Surg Res. 115:48–55. 2003.Balla G., Jacob HS., Balla J., Rosenberg M., Nath KA., Apple F., Eaton JW., Vercellotti GM. Ferritin: a cytoprotective antioxidant stratagem of endothelium. J Biol Chem. 267:18148–18153. 1992.Balla J., Jacob HS., Balla G., Nath KA., Eaton JW., Vercellotti GM. Endothelial cell heme uptake from heme proteins: induction of sensitization and desensitization to oxidant damage. Proc Natl Acad Sci USA. 90:9285–9289. 1993.Balla J., Nath KA., Balla G., Juckett MB., Jacob HS., Vercellotti GM. Endothelial cell heme oxygenase and ferritin induction in rat lung by hemoglobin in vivo. Am J Physiol. 268:L321–327. 1995.
ArticleBerberat PO., Katori M., Kaczmarek E., Anselmo D., Lassman C., Ke B., Shen X., Busuttil RW., Yamashita K., Csizmadia E., Tyagi S., Otterbein LE., Brouard S., Tobiasch E., Bach FH., Kupiec- Weglinski JW., Soares MP. Heavy chain ferritin acts as an antiapoptotic gene that protects livers from ischemia reperfusion injury. FASEB J. 17:1724–1726. 2003.Cairo G., Tacchini L., Pogliaghi G., Anzon E., Tomasi A., Bernelli-Zazzera A. Induction of ferritin synthesis by oxidative stress. Transcriptional and post-transcriptional regulation by expansion of the “free” iron pool. J Biol Chem. 270:700–703. 1995.Carraway MS., Ghio AJ., Taylor JL., Piantadosi CA. Induction of ferritin and heme oxygenase-1 by endotoxin in the lung. Am J Physiol. 275:L583–592. 1998.Choi AM., Alam J. Heme oxygenase-1: Function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 15:9–19. 1996.
ArticleConnelly KG., Moss M., Parsons PE., Moore EE., Moore FA., Giclas PC., Seligman PA., Repine JE. Serum ferritin as a predictor of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 155:21–25. 1997.
ArticleEisenstein RS., Garcia-Mayol D., Pettingell W., Munro HN. Regulation of ferritin and heme oxygenase synthesis in rat fibroblasts by different forms of iron. Proc Natl Acad Sci USA. 88:688–692. 1991.
ArticleFerris CD., Jaffrey SR., Sawa A., Takahashi M., Brady SD., Barrow RK., Tysoe SA., Wolosker H., Baranano DE., Dore S., Poss KD., Snyder SH. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol. 1:152–157. 1999.
ArticleGabay C., Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 340:448–454. 1999.
ArticleGrace PA. Ischaemia-reperfusion injury. Br J Surg. 81:637–647. 1994.
ArticleHalliwell B. Oxidants and human diseases: some new concepts. FASEB J. 1:358–364. 1987.Harrison PM., Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta. 1275:161–203. 1996.
ArticleHassoun HT., Kozar RA., Kone BC., Safi HJ., Moore FA. Intraischemic hypothermia differentially modulates oxidative stress proteins during mesenteric ischemia/reperfusion. Surgery. 132:369–376. 2002.
ArticleJacobs A., Worwood M. Ferritin in serum. Clinical and biochemical implications. N Engl J Med. 292:951–956. 1975.Ji YS., Kim NH., Jung HG., Ha DY., Jung KH. Siginificance of serum ferritin in multiple trauma patients with acute respiratory distress syndrome. J Korean Soc Traumatol. 20:57–64. 2007.Juckett MB., Balla J., Balla G., Jessurun J., Jacob HS., Vercellotti GM. Ferritin protects endothelial cells from oxidized low density lipoprotein in vitro. Am J Pathol. 147:782–789. 1995.Kakhlon O., Gruenbaum Y., Cabantchik ZL. Repression of the heavy ferritin chain increases the labile iron pool of human K562 cells. Biochem J. 356:311–316. 2001.
ArticleKato H., Amersi F., Buelow R., Melinek J., Coito AJ., Ke B., Busuttil RW., Kupiec-Weglinski JW. Heme oxygenase-1 overexpression protects rat livers from ischemia/reperfusion injury with extended cold preservation. Am J Transplant. 1:121–128. 2001.
ArticleKatori M., Buelow R., Ke B., Ma J., Coito AJ., Iyer S., Southard D., Busuttil RW., Kupiec-Weglinski JW. Heme oxygenase-1 over-expression protects rat hearts from cold ischemia/reperfusion injury via an anti-apoptotic pathway. Transplantation. 73:287–292. 2002.Kennedy TP., Rao NV., Hopkins C., Pennington L., Tolley E., Hoidal JR. Role of reactive oxygen species in reperfusion injury of the rabbit lung. J Clin Invest. 83:1326–1335. 1989.
ArticleKim YM., Bergonia H., Lancaster JR Jr. Nitrogen oxide-induced autoprotection in isolated rat hepatocytes. FEBS Lett. 374:228–232. 1995.
ArticleKlausner RD., Rouault TA., Harford JB. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 72:19–28. 1993.
ArticleLee PJ., Alam J., Weigand GW., Choi AMK. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc Natl Acad Sci USA. 93:10393–10398. 1996.
ArticleLee PJ., Camhi SL., Chin BY., Alam J., Choi AM. AP-1 and STAT mediate hyperoxia-induced gene transcription of heme oxygenase-1. Am J Physiol Lung Cell Mol Physiol. 279:L175–182. 2000.
ArticleLi N., Venkatesan MI., Miguel A., Kaplan R., Gujuluva C., Alam J., Nel A. Induction of heme oxygenase-1 expression in macro-phages by diesel exhaust particle chemicals and quinones via the antioxidant-responsive element. Immunol. 165:3393–3401. 2000.
ArticleMinamiya Y., Tozawa K., Kitamura M., Saito S., Ogawa J. Platelet-activating factor mediates intercellular adhesion molecule-1-dependent radical production in the nonhypoxic ischemia rat lung. Am J Respir Cell Mol Biol. 19:150–157. 1998.
ArticleMumby S., Upton RL., Chen Y., Stanford SJ., Quinlan GJ., Nicholson AG., Gutteridge JM., Lamb NJ., Evans TW. Lung heme oxygenase-1 is elevated in acute respiratory distress syndrome. Crit Care Med. 32:1130–1135. 2004.
ArticleOtamiri T., Lindahl M., Tagasson C. Phospholipase A2 inhibition prevents mucosal damage associated with small intestine ischemia in rats. Gut. 29:489–494. 1988.Otterbein L., Chin BY., Otterbein SL., Lowe VC., Fessler HE., Choi AM. Mechanism of hemoglobin-induced protection against endotoxemia in rats: a ferritin-independent pathway. Am J Physiol. 272:L268–275. 1997.
ArticleOtterbein L., Sylvester SL., Choi AMK. Hemoglobin provides protection against lethal endotoxemia in rats: the role of heme oxygenase-1. Am J Respir Cell Mol Biol. 13:595–601. 1995.
ArticleOtterbein LE., Soares MP., Yamashita K., Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 24:449–455. 2003.
ArticlePark SD., Park YY. Changes of serum ferritin in acute lung injury induced by intestinal ischemia/reperfusion. Korean J Physiol Pharmacol. 10:187–191. 2006.Park YY., Lee YM. Effects of aspirin on the pathogenesis of acute lung injury in rats subjected to hemorrhage. Tuberc Respir Dis. 60:83–91. 2006.
ArticlePark YY., Hybertson BM., Wright RM., Fini MA., Elkins ND., Repine JE. Serum ferritin elevation and acute lung injury in rats subjected to hemorrhage: Reduction by mepacrine treatment. Exp Lung Res. 30:571–584. 2004.
ArticlePark YY., Hybertson BM., Wright RM., Repine JE. Serum ferritin increases in hemorrhaged rats that develop acute lung injury: effect of an iron-deficient diet. Inflammation. 27:257–263. 2003.Picard V., Epsztejn S., Santambrogio P., Cabantchik ZI., Beaumont C. Role of ferritin in the control of the labile iron pool in murine erythroleukemia cells. J Biol Chem. 273:15382–15386. 1998.
ArticleRepine JE. Scientific perspectives on adult respiratory distress syndrome. Lancet. 339:466–469. 1992.
ArticleRogers JT., Bridges KR., Durmowicz GP., Glass J., Auron PE., Munro HN. Translational control during the acute phase response. Ferritin synthesis in response to interleukin-1. J Biol Chem. 265:14572–14578. 1990.
ArticleRyter SW., Alarm J., Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 86:583–650. 2006.
ArticleScott JR., Chin BY., Bilbana MH., Otterbein LE. Restoring HOmeostasis: is heme oxygenase-1 ready for the clinic? Trends Pharmacol Sci. 28:200–205. 2007.
ArticleSharkey RA., Donnelly SC., Connelly KG., Robertson CE., Haslett C., Repine JE. Initial serum ferritin levels in patients with multiple trauma and the subsequent development of acute respiratory distress syndrome. Am J Respir Crit Care Med. 159:1506–1509. 1999.
ArticleSoncul H., Oz E., Kalaycioglu S. Role of ischemic preconditioning on ischemia-reperfusion injury of the lung. Chest. 115:1672–1677. 1999.
ArticleTacchini L., Recalcati S., Bernelli-Zazzera A., Cairo G. Induction of ferritin synthesis in ischemic reperfused rat liver: analysis of the molecular mechanisms. Gastroenterology. 113:946–953. 1997.Taylor JL., Carraway MS., Piantadosi CA. Lung-specific induction of heme oxygenase-1 and hyperoxic lung injury. Am J Physiol. 274:L582–590. 1998.Tenhunen R., Marver HS., Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 61:748–755. 1968.
ArticleTorti FM., Torti SV. Regulation of ferritin genes and protein. Blood. 99:3505–3516. 2002.
ArticleVile GF., Basu-Modak S., Waltner C., Tyrrell RM. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci USA. 91:2607–2610. 1994.
ArticleWare LB., Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 324:1334–1349. 2000.
ArticleWillis D., Moore AR., Frederick R., Willoughby DA. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat Med. 2:87–90. 1996.Yang F., Coalson JJ., Bobb HH., Carter JD., Banu J., Ghio AJ. Resistance of hypotransferrinemic mice to hyperoxia-induced lung injury. Am J Physiol. 277:L1214–1223. 1999.Yet SF., Pellacani A., Patterson C., Tan L., Folta SC., Foster L., Lee WS., Hsieh CM., Perrella MA. Induction of heme oxygenase-1 expression in vascular smooth muscle cells: a link to endotoxic shock. J Biol Chem. 272:4295–4301. 1997.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Changes of Serum Ferritin in Acute Lung Injury Induced by Intestinal Ischemia/Reperfusion
- Low Dose Carbon Monoxide Inhalation Prevents Chronic Allograft Nephropathy following Kidney Transplantation in Rats. Heme Oxygenase-1 Derivatives Study I
- Protective Effects of Geniposide and Genipin against Hepatic Ischemia/Reperfusion Injury in Mice
- Heme Oxygenase-1: Its Therapeutic Roles in Inflammatory Diseases
- Effects of Aspirin on the Pathogenesis of Acute Lung Injury in Rats Subjected to Hemorrhage