Tuberc Respir Dis.
2006 Jan;60(1):83-91.
Effects of Aspirin on the Pathogenesis of Acute Lung Injury in Rats Subjected to Hemorrhage
- Affiliations
-
- 1Department of Physiology, Catholic University of Daegu, Daegu, Korea. yypark@cu.ac.kr
Abstract
-
BACKGROUND: For unknown reasons, the serum ferritin concentrations are higher in patients with acute lung injury. A pretreatment with aspirin reduces the acute lung injury in rats subjected severe hemorrhage, and increases the rate of ferritin synthesis in vitro. This study investigated the effect of aspirin on the serum ferritin changes in rats subjected to severe hemorrhage.
METHODS
Hemorrhagic shock was induced by withdrawing blood (20 ml/kg of B.W.) through the femoral artery for 5 min. The rats were pretreated with aspirin (10 mg/kg, i.v.) 30 min before hemorrhage.
RESULTS
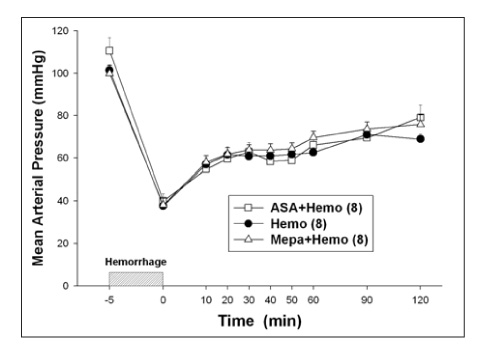
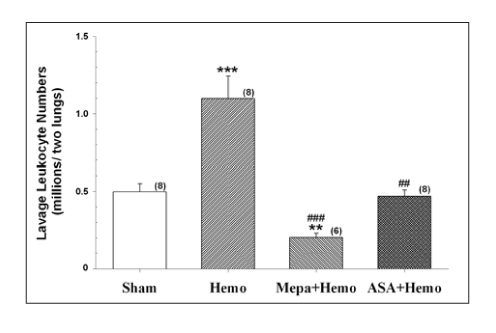
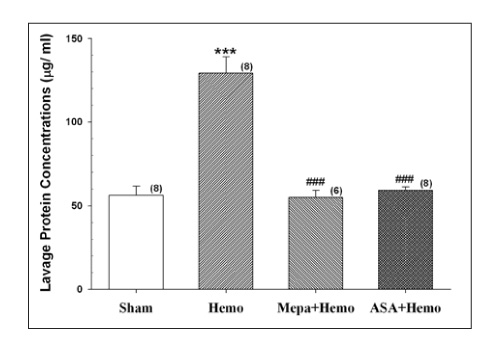
The protein content and leukocyte count in the bronchoalveolar lavage fluid, lung tissue myeloperoxidase activities were significantly higher after hemorrhage. The aspirin pretreatment prevented these changes. The serum and lavage fluid ferritin concentrations were elevated higher after hemorrhage. These were also attenuated by the aspirin pretreatment.
CONCLUSION
The changes in the serum and lung lavage ferritin level might be closely related to the severity of hemorrhage?induced acute lung injury. Therefore, the serum and lavage ferritin concentrations can be a useful biomarker for patients with precipitating conditions.
Keyword
MeSH Terms
Figure
Reference
-
1. Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim Biophys Acta. 1996. 1275:161–203.2. Torti FM, Torti SV. Regulation of ferritin genes and protein. Blood. 2002. 99:3505–3516.3. Arosio P, Levi S. Ferritin, iron homeostasis, and oxidative damage. Free Radic Biol Med. 2002. 33:457–463.4. Balla G, Jacob HS, Balla J, Rosenberg M, Nath K, Apple F, et al. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992. 267:18148–18153.5. Hybertson BM, Connelly KG, Buser RT, Repine JE. Ferritin and desferrioxamine attenuate xanthine oxidase-dependent leak in isolated perfused rat lungs. Inflammation. 2002. 26:153–159.6. Park YY, Hybertson BM, Wright RM, Repine JE. Serum ferritin increases in hemorrhaged rats that develop acute lung injury: effect of an iron-deficient diet. Inflammation. 2003. 27:257–263.7. Park YY, Hybertson BM, Wright RM, Fini MA, Elkins N, Repine JE. Serum ferritin elevation and acute lung injury in rats subjected to hemorrhage: reduction by mepacrine treatment. Exp Lung Res. 2004. 30:571–584.8. Connelly KG, Moss M, Parsons PE, Moore EE, Moore FA, Giclas PC, et al. Serum ferritin as a predictor of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997. 155:21–25.9. Sharkey RA, Donnelly SC, Connelly KG, Robertson CE, Haslett C, Repine JE. Initial serum ferritin levels in patients with multiple trauma and the subsequent development of acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999. 159:1506–1509.10. Pham CG, Bubici C, Zazzeroni F, Papa S, Jones J, Alvarez K, et al. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell. 2004. 119:529–542.11. Podhaisky HP, Abate A, Polte T, Oberle S, Schroder H. Aspirin protects endothelial cells from oxidative stress: possible synergism with vitamin E. FEBS Lett. 1997. 417:349–351.12. Wahn H, Hammerschmidt S. Inhibition of PMN- and HOCl-induced vascular injury in isolated rabbit lungs by acetylsalicylic acid: a possible link between neutrophil-derived oxidative stress and eicosanoid metabolism? Biochim Biophys Acta. 1998. 1408:55–66.13. Carpenter-Deyo L, Roth RA. Cyclooxygenase inhibition in lungs or in neutrophils attenuates neutrophil-dependent edema in rat lungs perfused with phorbol myristate acetate. J Pharmacol Exp Ther. 1989. 251:983–991.14. Zanaboni PB, Bradley JD, Baudendistel LJ, Webster RO, Dahms TE. Cyclooxygenase inhibition prevents PMA-induced increases in lung vascular permeability. J Appl Physiol. 1990. 69:1494–1501.15. Shin TR, Lee DU, Park YY. Aspirin reduces acute lung injury in rats subjected to severe hemorrhage. Tuberc Respir Dis. 2003. 54:522–531.16. Kopp E, Ghosh S. Inhibition of NF-kappa B by sodium salicylate and aspirin. Science. 1994. 265:956–959.17. Yin MJ, Yamamoto Y, Gaynor RB. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature. 1998. 396:77–80.18. Eisele G, Schwedhelm E, Schieffer B, Tsikas D, Boger RH. Acetylsalicylic acid inhibits monocyte adhesion to endothelial cells by an antioxidative mechanism. J Cardiovasc Pharmacol. 2004. 43:514–521.19. Brown RE, Jarvis KL, Hyland KJ. Protein measurement using bicinchoninic acid: elimination of interfering substance. Anal Biochem. 1989. 180:136–139.20. Goldblum SE, Wu KM, Jay M. Lung myeloperoxidase as a measure of leukostasis in rabbit. J Appl Physiol. 1985. 59:1978–1985.21. Jang YS, Kim SE, Jheon SH, Shin TR, Lee YM. Phospholipase A2 contributes to hemorrhage-induced acute lung injury through neutrophilic respiratory burst. Tuberc Respir Dis. 2001. 51:503–516.22. Repine JE. Scientific perspectives on adult respiratory distress syndrome. Lancet. 1992. 339:466–469.23. Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for the development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995. 151:293–301.24. Hierholzer C, Harbrecht B, Menezes JM, Kane J, MacMicking J, Nathan CF, et al. Essential role of induced nitric oxide in the initiation of the inflammatory response after hemorrhagic shock. J Exp Med. 1998. 187:917–928.25. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000. 342:1334–1349.26. Connelly KG, Repine JE. Markers for predicting the development of acute respiratory distress syndrome. Annu Rev Med. 1997. 48:429–445.27. Pepe PE, Potkin RT, Reus DH, Hudson LD, Carrico CJ. Clinical predictors of the adult respiratory distress syndrome. Am J Surg. 1982. 144:124–130.28. Fowler AA, Hamman RF, Good JT, Benson KN, Baird M, Eberle DJ, et al. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med. 1983. 98:593–597.29. Halliwell B, Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984. 219:1–14.30. Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989. 320:365–376.31. Gannon DE, Varani J, Phan SH, Ward JH, Kaplan J, Till GO, et al. Source of iron in neutrophil-mediated killing of endothelial cells. Lab Invest. 1987. 57:37–44.32. Schraufstatter I, Hyslop PA, Jackson JH, Cochrane CG. Oxidant-induced DNA damage of target cells. J Clin Invest. 1988. 82:1040–1050.33. Yang F, Coalson JJ, Bobb HH, Carter JD, Banu J, Ghio AJ. Resistance of hypotransferrinemic mice to hyperoxia-induced lung injury. Am J Physiol. 1999. 277:L1214–L1223.34. Rogers JT, Bridges KR, Durmowicz GP, Glass J, Auron PE, Munro HN. Translational control during the acute phase response: ferritin synthesis in response to interleukin-1. J Biol Chem. 1990. 265:14572–14578.35. Tran TN, Eubanks SK, Schaffer KJ, Zhou CY, Linder MC. Secretion of ferritin by rat hepatoma cells and its regulation by inflammatory cytokines and iron. Blood. 1997. 90:4979–4986.36. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999. 340:448–454.37. Cook JD, Lipschitz DA, Miles LE, Finch CA. Serum ferritin as a measure of iron stores in normal subjects. Am J Clin Nutr. 1974. 27:681–687.38. Walters GO, Miller FM, Worwood M. Serum ferritin concentration and iron stores in normal subjects. J Clin Pathol. 1973. 26:770–772.39. Zweifach BW. Mechanisms of blood flow and fluid exchange in microvessels: hemorrhagic hypotension model. Anesthesiology. 1974. 41:157–168.40. Jacobs A, Worwood M. Ferritin in serum: clinical and biochemical implications. N Engl J Med. 1975. 292:951–956.41. McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985. 312:159–163.42. Anderson BO, Moore EE, Moore FA, Leff JA, Terada LS, Harken AH, et al. Hypovolemic shock promotes neutrophil sequestration in lungs by a xanthine oxidase-related mechanism. J Appl Physiol. 1991. 71:1862–1865.43. Tan S, Yokoyama Y, Dickens E, Cash TG, Freeman BA, Parks DA. Xanthine oxidase activity in the circulation of rats following hemorrhagic shock. Free Radic Biol Med. 1993. 15:407–414.44. Schwartz MD, Repine JE, Abraham E. Xanthine oxidase-derived oxygen radicals increase lung cytokine expression in mice subjected to hemorrhagic shock. Am J Respir Cell Mol Biol. 1995. 12:434–440.45. Balla J, Nath KA, Balla G, Juckett MB, Jacob HS, Vercellotti GM. Endothelial cell heme oxygenase and ferritin induction in rat lung by hemoglobin in vivo. Am J Physiol. 1995. 268:L321–L327.46. Kwak EL, Larochelle DA, Beaumont C, Torti SV, Torti FM. Role for NF-kappa B in the regulation of ferritin H by tumor necrosis factor-alpha. J Biol Chem. 1995. 270:15285–15293.47. Holter JF, Weiland JE, Pacht ER, Gadek JE, Davis WB. Protein permeability in the adult respiratory distress syndrome: loss of size selectivity of the alveolar epithelium. J Clin Invest. 1986. 78:1513–1522.48. Suter PM, Suter S, Girardin E, Roux-Lombard P, Grau GE, Dayer JM. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. Am Rev Respir Dis. 1992. 145:1016–1022.49. Shenkar R, Abraham E. Mechanisms of lung neutrophil activation after hemorrhage or endotoxemia: roles of reactive oxygen intermediates, NF-kappa B, and cyclic AMP response element binding protein. J Immunol. 1999. 163:954–962.50. Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhage-or endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2000. 279:L1137–L1145.51. Tsuji Y, Miller LL, Miller SC, Torti SV, Torti FM. Tumor necrosis factor-alpha and interleukin 1-alpha regulate transferrin receptor in human diploid fibroblasts: relationship to the induction of ferritin heavy chain. J Biol Chem. 1991. 266:7257–7261.52. Reif DW. Ferritin as a source of iron for oxidative damage. Free Radic Biol Med. 1992. 12:417–427.53. Balla G, Vercellotti GM, Eaton JW, Jacob HS. Iron loading of endothelial cells augments oxidant damage. J Lab Clin Med. 1990. 116:546–554.54. Olakanmi O, McGowan SE, Hayek MB, Britigan BE. Iron sequestration by macrophages decreases the potential for extracellular hydroxyl radical formation. J Clin Invest. 1993. 91:889–899.55. Orino K, Lehman L, Tsuji Y, Ayaki H, Torti SV, Torti FM. Ferritin and the response to oxidative stress. Biochem J. 2001. 357:241–247.56. Oberle S, Polte T, Abate A, Podhaisky HP, Schroder H. Aspirin increases ferritin synthesis in endothelial cells: a novel antioxidant pathway. Circ Res. 1998. 82:1016–1020.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Aspirin Reduces Acute Lung Injury in Rats Subjected to Severe Hemorrhage
- Phospholipase A2 Contributes to Hemorrhage-induced Acute Lung Injury Through Neutrophilic Respiratory Burst
- Role of the PLA2-Activated Neutrophilic Oxidative Stress in Oleic Acid-Induced Acute Lung Injury
- Effect of Neutrophil Elastase inhibitor, ICI 200,355, on Interleukin-1 Induced acute lung injury in rats
- Antiinflammatory Effects of Heparin in Hemorrhage or LPS Induced Acute Lung Injury