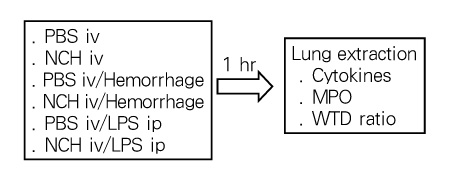
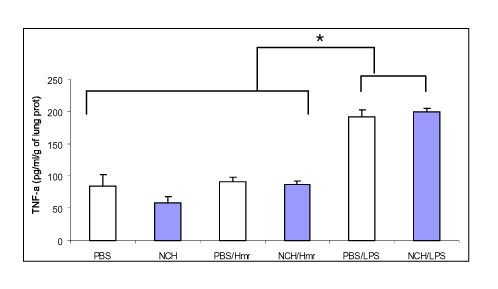
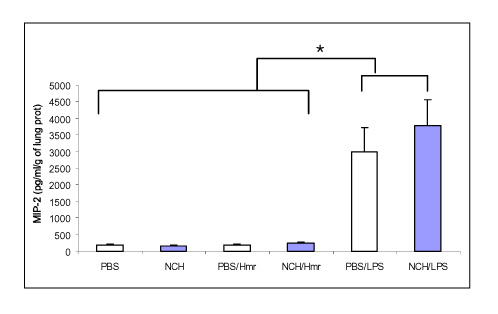
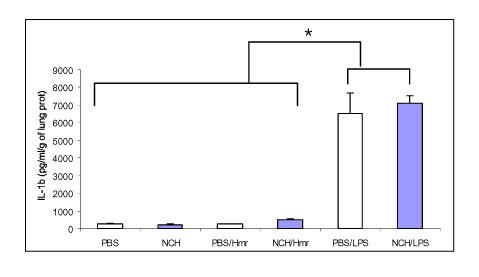
Tuberc Respir Dis.
2006 Jan;60(1):49-56.
Antiinflammatory Effects of Heparin in Hemorrhage or LPS Induced Acute Lung Injury
- Affiliations
-
- 1Department of Internal Medicine, Chung-Ang University College of Medicine, Korea. jykimmd@cau.ac.kr
Abstract
- No abstract available.
MeSH Terms
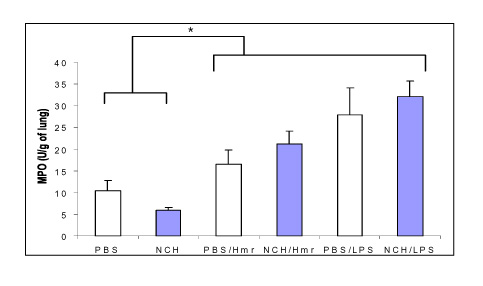
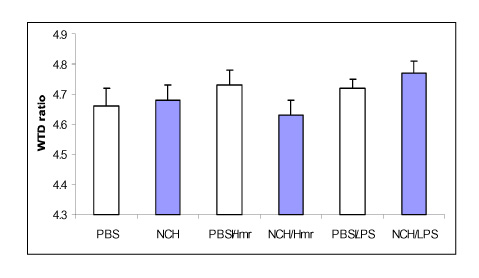
Figure
Reference
-
1. Repine JE. Scientific perspectives on adult respiratory distress syndrome. Lancet. 1992. 339:466–469.2. Kollef MH, Schuster DP. The acute respiratory distress syndrome. N Engl J Med. 1995. 332:27–37.3. Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000. 342:1334–1349.4. Chollet-Martin S, Jourdain B, Gibert C, Elbim C, Chastre J, Gougerot-Pocidalo MA. Interactions between neutrophils and cytokines in blood and alveolar spaces during ARDS. Am J Respir Crit Care Med. 1996. 154:594–601.5. Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, et al. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1996. 154:602–611.6. Suter PM, Suter S, Girardin E, Roux-Lombard P, Grau GE, Dayer JM. High bronchoalveolar levels of tumor necrosis factor and its inhibitors, interleukin-1, interferon, and elastase, in patients with adult respiratory distress syndrome after trauma, shock, or sepsis. Am Rev Respir Dis. 1992. 145:1016–1022.7. Abraham E, Carmody A, Shenkar R, Arcaroli J. Neutrophils as early immunologic effectors in hemorrhageor endotoxemia-induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2000. 279:L1137–L1145.8. Parsey MV, Tuder RM, Abraham E. Neutrophils are major contributors to intraparenchymal lung IL-1 beta expression after hemorrhage and endotoxemia. J Immunol. 1998. 160:1007–1013.9. Shenkar R, Abraham E. Mechanisms of lung neutrophil activation after hemorrhage or endotoxemia: roles of reactive oxygen intermediates, NF-kappa B, and cyclic AMP response element binding protein. J Immunol. 1999. 163:954–962.10. Xing Z, Jordana M, Kirpalani H, Driscoll KE, Schall TJ, Gauldie J. Cytokine expression by neutrophils and macrophages in vivo: endotoxin induces tumor necrosis factor-alpha, macrophage inflammatory protein-2, interleukin-1 beta, and interleukin-6 but not RANTES or transforming growth factor-beta 1 mRNA expression in acute lung inflammation. Am J Respir Cell Mol Biol. 1994. 10:148–153.11. Jaques LB. Heparins: anionic polyelectrolyte drugs. Pharmacol Rev. 1979. 31:99–166.12. Rao NV, Kennedy TP, Rao G, Ky N, Hoidal JR. Sulfated polysaccharides prevent human leukocyte elastase-induced acute lung injury and emphysema in hamsters. Am Rev Respir Dis. 1990. 142:407–412.13. Weiler JM, Edens RE, Linhardt RJ, Kapelanski DP. Heparin and modified heparin inhibit complement activation in vivo. J Immunol. 1992. 148:3210–3215.14. Hocking DC, Ferro TJ, Johnson A. Dextran sulfate inhibits PMN-dependent hydrostatic pulmonary edema induced by tumor necrosis factor. J Appl Physiol. 1991. 70:1121–1128.15. Skinner MP, Lucas CM, Burns GF, Chesterman CN, Berndt MC. GMP-140 binding to neutrophils is inhibited by sulfated glycans. J Biol Chem. 1991. 266:5371–5374.16. Ley K, Cerrito M, Arfors KE. Sulfated polysaccharides inhibit leukocyte rolling in rabbit mesentery venules. Am J Physiol. 1991. 260:H1667–H1673.17. Simon RH, DeHart PD, Todd RF 3rd. Neutrophil-induced injury of rat pulmonary alveolar epithelial cells. J Clin Invest. 1986. 78:1375–1386.18. Inoue Y, Nagasawa K. Selective N-desulfation of heparin with dimethyl sulfoxide containing water or methanol. Carbohydr Res. 1976. 46:87–95.19. Fryer A, Huang YC, Rao G, Jacoby D, Mancilla E, Whorton R, et al. Selective O-desulfation produces nonanticoagulant heparin that retains pharmacological activity in the lung. J Pharmacol Exp Ther. 1997. 282:208–219.20. Thourani VH, Brar SS, Kennedy TP, Thornton LR, Watts JA, Ronson RS, et al. Nonanticoagulant heparin inhibits NF-kappaB activation and attenuates myocardial reperfusion injury. Am J Physiol Heart Circ Physiol. 2000. 278:H2084–H2093.21. Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001. 29:1303–1310.22. Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003. 348:1546–1554.23. Rainer TH, Lam PK, Wong EM, Cocks RA. Derivation of a prediction rule for post-traumatic acute lung injury. Resuscitation. 1999. 42:187–196.24. McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985. 312:159–163.25. Deitch EA. Multiple organ failure: pathophysiology and potential future therapy. Ann Surg. 1992. 216:117–134.26. Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS): a prospective study. JAMA. 1995. 273:117–123.27. Sands KE, Bates DW, Lanken PN, Graman PS, Hibberd PL, Kahn KL, et al. Epidemiology of sepsis syndrome in 8 academic medical centers. JAMA. 1997. 278:234–240.28. Ayala A, Perrin MM, Meldrum DR, Ertel W, Chaudry IH. Hemorrhage induces an increase in serum TNF which is not associated with elevated level of endotoxin. Cytokine. 1990. 2:170–174.29. Hierholzer C, Kalff JC, Omert L, Tsukada K, Loeffert JE, Watkins SC, et al. Interleukin-6 production in hemorrhagic shock is accompanied by neutrophil recruitment and lung injury. Am J Physiol. 1998. 275:L611–L621.30. Friedrichs GS, Kilgore KS, Manley PJ, Gralinski MR, Lucchesi BR. Effects of heparin and N-acetyl heparin on ischemia/reperfusion-induced alterations in myocardial function in the rabbit isolated heart. Circ Res. 1994. 75:701–710.31. Black SC, Gralinski MR, Friedrichs GS, Kilgore KS, Driscoll EM, Lucchesi BR. Cardioprotective effects of heparin or N-acetylheparin in an in vivo model of myocardial ischaemic and reperfusion injury. Cardiovasc Res. 1995. 29:629–636.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diosmetin Alleviates Lipopolysaccharide-Induced Acute Lung Injury through Activating the Nrf2 Pathway and Inhibiting the NLRP3 Inflammasome
- The Role of Keratinocyte-derived Chemokine in Hemorrhage-induced Acute Lung Injury in Mice
- Effects of Aspirin on the Pathogenesis of Acute Lung Injury in Rats Subjected to Hemorrhage
- Aspirin Reduces Acute Lung Injury in Rats Subjected to Severe Hemorrhage
- The Efficacy of alpha-lipoic Acid on the Endotoxin-induced Acute Lung Injury