Nutr Res Pract.
2014 Jun;8(3):257-266.
Anti-carcinogenic effects of non-polar components containing licochalcone A in roasted licorice root
- Affiliations
-
- 1Department of Food Science and Nutrition, Hallym University, 1 Hallymdaehak-gil, Gangwon 200-702, Korea. jyoon@hallym.ac.kr
- 2Advanced Institutes of Convergence Technology, Seoul National University, Suwon, Gyeonggi 443-270, Korea.
- 3Center for Efficacy Assessment and Development of Functional Foods and Drugs, Hallym University, Gangwon 200-702, Korea.
- 4Center for Food and Nutritional Genomics Research and Department of Food Science and Nutrition, Kyungpook National University, Daegu 702-701, Korea.
- 5WCU Biomodulation Major, Department of Agricultural Biotechnology and Center for Food and Bioconvergence, Seoul National University, Seoul 151-921, Korea.
Abstract
- BACKGROUND
/OBJECTIVE: Licorice has been shown to possess cancer chemopreventive effects. However, glycyrrhizin, a major component in licorice, was found to interfere with steroid metabolism and cause edema and hypertension. The roasting process of licorice modifies the chemical composition and converts glycyrrhizin to glycyrrhetinic acid. The purpose of this study was to examine the anti-carcinogenic effects of the ethanol extract of roasted licorice (EERL) and to identify the active compound in EERL.
MATERIALS/METHODS
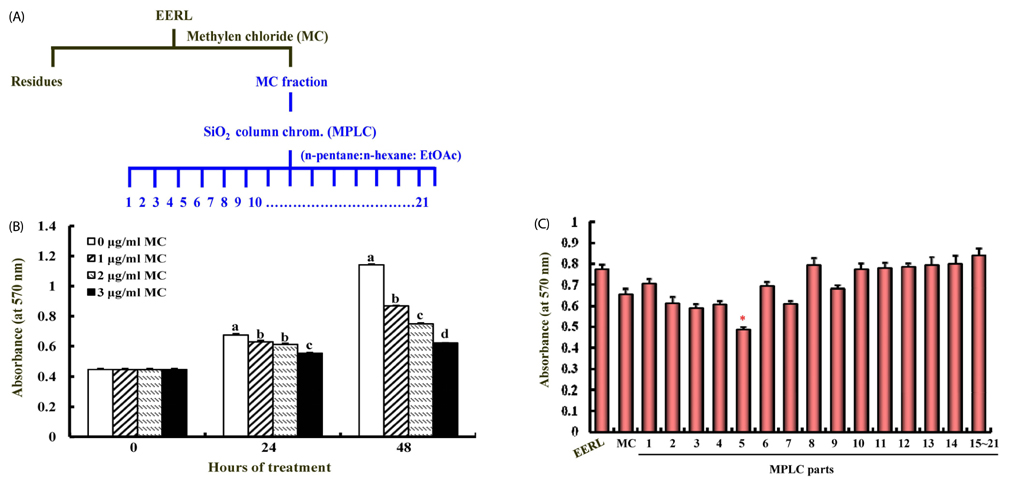
Ethanol and aqueous extracts of roasted and un-roasted licorice were prepared. The active fraction was separated from the methylene chloride (MC)-soluble fraction of EERL and the structure of the purified compound was determined by nuclear magnetic resonance spectroscopy. The anti-carcinogenic effects of licorice extracts and licochalcone A was evaluated using a MTT assay, Western blot, flow cytometry, and two-stage skin carcinogenesis model.
RESULTS
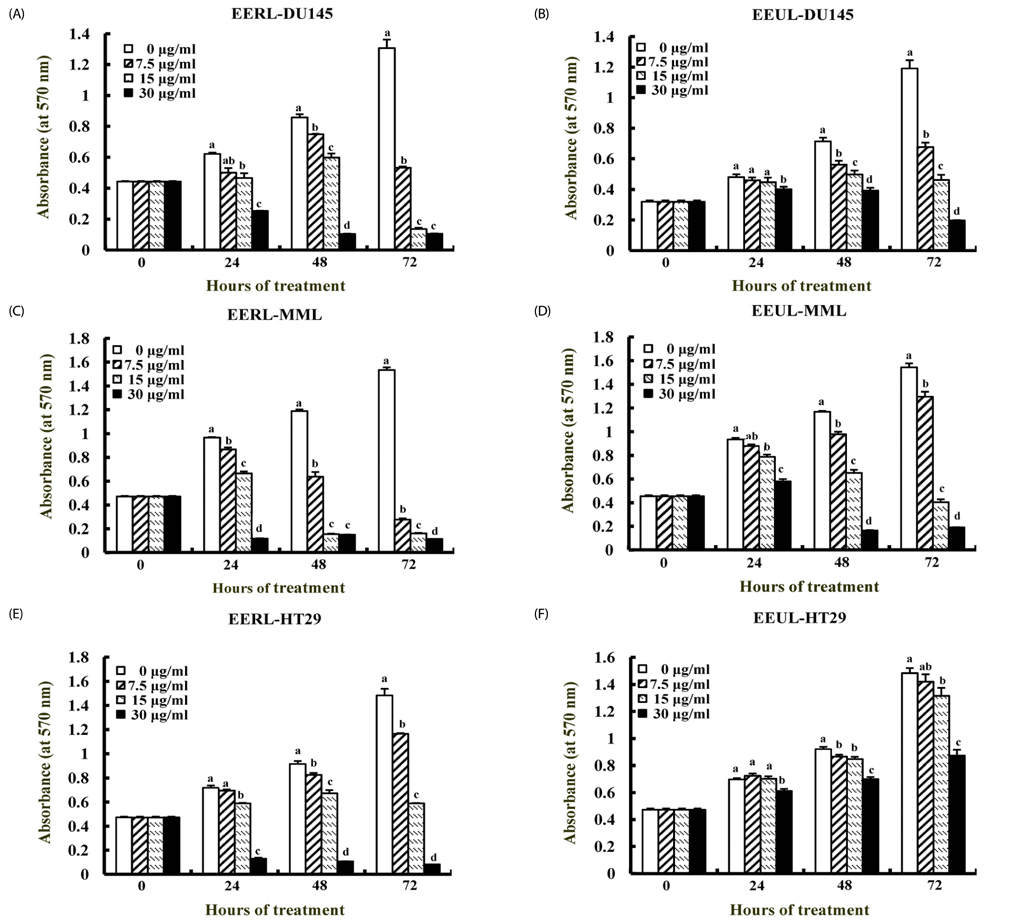
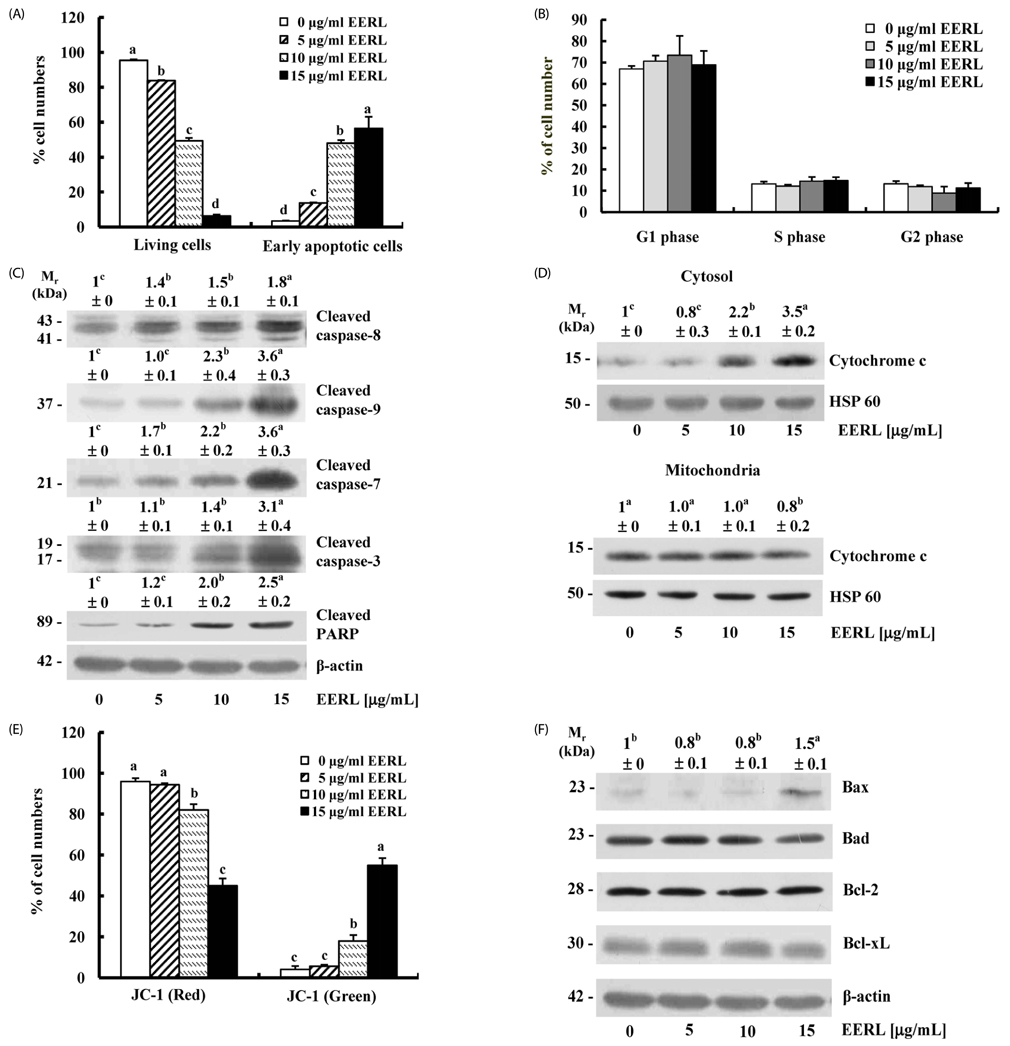
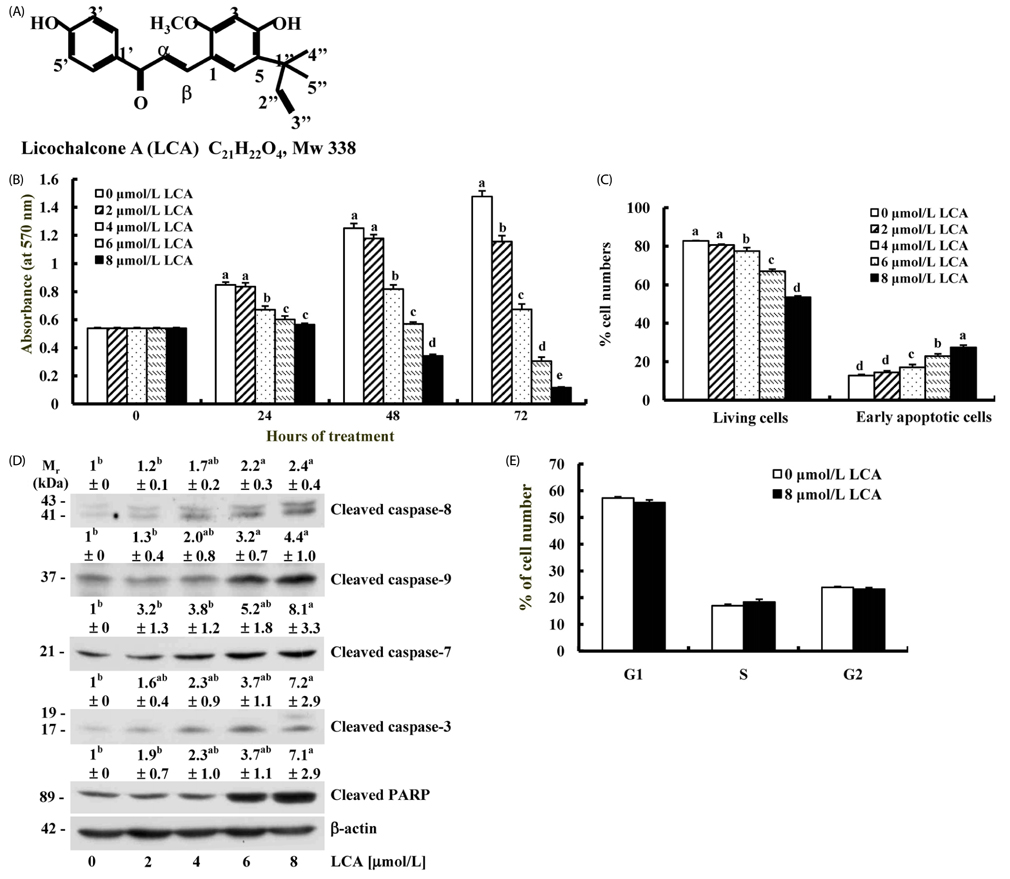
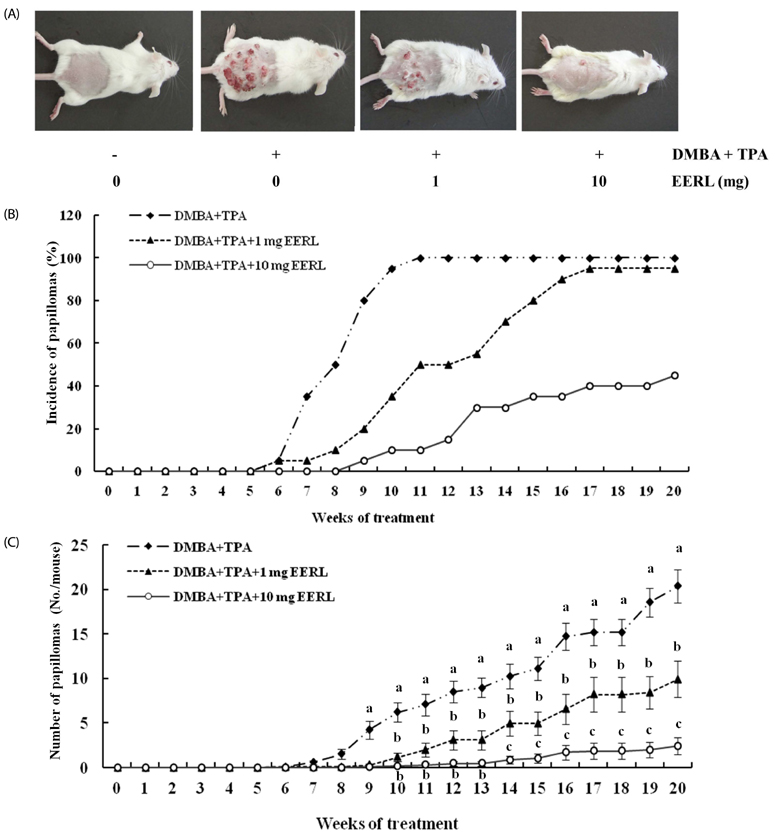
EERL was determined to be more potent and efficacious than the ethanol extract of un-roasted licorice in inhibiting the growth of DU145 and MLL prostate cancer cells, as well as HT-29 colon cancer cells. The aqueous extracts of un-roasted and roasted licorice showed minimal effects on cell growth. EERL potently inhibited growth of MCF-7 and MDA-MB-231 breast, B16-F10 melanoma, and A375 and A2058 skin cancer cells, whereas EERL slightly stimulated the growth of normal IEC-6 intestinal epithelial cells and CCD118SK fibroblasts. The MC-soluble fraction was more efficacious than EERL in inhibiting DU145 cell growth. Licochalcone A was isolated from the MC fraction and identified as the active compound of EERL. Both EERL and licochalcone A induced apoptosis of DU145 cells. EERL potently inhibited chemically-induced skin papilloma formation in mice.
CONCLUSIONS
Non-polar compounds in EERL exert potent anti-carcinogenic effects, and that roasted rather than un-roasted licorice should be favored as a cancer preventive agent, whether being used as an additive to food or medicine preparations.
Keyword
MeSH Terms
-
Animals
Anticarcinogenic Agents*
Apoptosis
Blotting, Western
Breast
Carcinogenesis
Colonic Neoplasms
Edema
Epithelial Cells
Ethanol
Fibroblasts
Flow Cytometry
Glycyrrhetinic Acid
Glycyrrhiza*
Glycyrrhizic Acid
Hypertension
Magnetic Resonance Spectroscopy
Melanoma
Metabolism
Methylene Chloride
Mice
Papilloma
Prostatic Neoplasms
Skin
Skin Neoplasms
Spectrum Analysis
Anticarcinogenic Agents
Ethanol
Glycyrrhetinic Acid
Glycyrrhizic Acid
Methylene Chloride
Figure
Reference
-
1. Ouédraogo M, Charles C, Ouédraogo M, Guissou IP, Stévigny C, Duez P. An overview of cancer chemopreventive potential and safety of proanthocyanidins. Nutr Cancer. 2011; 63:1163–1173.
Article2. Kinghorn AD, Chai HB, Sung CK, Keller WJ. The classical drug discovery approach to defining bioactive constituents of botanicals. Fitoterapia. 2011; 82:71–79.
Article3. Tan AC, Konczak I, Sze DM, Ramzan I. Molecular pathways for cancer chemoprevention by dietary phytochemicals. Nutr Cancer. 2011; 63:495–505.
Article4. Vermeulen K, Van Bockstaele DR, Berneman ZN. Apoptosis: mechanisms and relevance in cancer. Ann Hematol. 2005; 84:627–639.
Article5. Wang ZY, Nixon DW. Licorice and cancer. Nutr Cancer. 2001; 39:1–11.
Article6. Asl MN, Hosseinzadeh H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother Res. 2008; 22:709–724.
Article7. Yasue H, Itoh T, Mizuno Y, Harada E. Severe hypokalemia, rhabdomyolysis, muscle paralysis, and respiratory impairment in a hypertensive patient taking herbal medicines containing licorice. Intern Med. 2007; 46:575–578.
Article8. van Uum SH. Liquorice and hypertension. Neth J Med. 2005; 63:119–120.9. Leitolf H, Dixit KC, Higham CE, Brabant G. Licorice - or more? Exp Clin Endocrinol Diabetes. 2010; 118:250–253.
Article10. Omar HR, Komarova I, El-Ghonemi M, Fathy A, Rashad R, Abdelmalak HD, Yerramadha MR, Ali Y, Helal E, Camporesi EM. Licorice abuse: time to send a warning message. Ther Adv Endocrinol Metab. 2012; 3:125–138.
Article11. Murphy SC, Agger S, Rainey PM. Too much of a good thing: a woman with hypertension and hypokalemia. Clin Chem. 2009; 55:2093–2096.
Article12. Sung MW, Li PC. Chemical analysis of raw, dry-roasted, and honey-roasted licorice by capillary electrophoresis. Electrophoresis. 2004; 25:3434–3440.
Article13. Hwang IK, Lim SS, Choi KH, Yoo KY, Shin HK, Kim EJ, Yoon-Park JH, Kang TC, Kim YS, Kwon DY, Kim DW, Moon WK, Won MH. Neuroprotective effects of roasted licorice, not raw form, on neuronal injury in gerbil hippocampus after transient forebrain ischemia. Acta Pharmacol Sin. 2006; 27:959–965.
Article14. Majima T, Yamada T, Tega E, Sakurai H, Saiki I, Tani T. Pharmaceutical evaluation of liquorice before and after roasting in mice. J Pharm Pharmacol. 2004; 56:589–595.
Article15. Kim KR, Jeong CK, Park KK, Choi JH, Park JH, Lim SS, Chung WY. Anti-inflammatory effects of licorice and roasted licorice extracts on TPA-induced acute inflammation and collagen-induced arthritis in mice. J Biomed Biotechnol. 2010; 2010:709378.
Article16. Jung JI, Lim SS, Choi HJ, Cho HJ, Shin HK, Kim EJ, Chung WY, Park KK, Park JH. Isoliquiritigenin induces apoptosis by depolarizing mitochondrial membranes in prostate cancer cells. J Nutr Biochem. 2006; 17:689–696.
Article17. Cho HJ, Kim WK, Kim EJ, Jung KC, Park S, Lee HS, Tyner AL, Park JH. Conjugated linoleic acid inhibits cell proliferation and ErbB3 signaling in HT-29 human colon cell line. Am J Physiol Gastrointest Liver Physiol. 2003; 284:G996–G1005.18. Eguchi Y, Srinivasan A, Tomaselli KJ, Shimizu S, Tsujimoto Y. ATP-dependent steps in apoptotic signal transduction. Cancer Res. 1999; 59:2174–2181.19. Wang B, Zou K, Yang XB, He WY, Zhao YY, Zhang RY. Two new flavanone glycosides from Glycyrrhizia inflata. Yao Xue Xue Bao. 1997; 32:199–202.20. Yo YT, Shieh GS, Hsu KF, Wu CL, Shiau AL. Licorice and licochalcone-a induce autophagy in LNCaP prostate cancer cells by suppression of Bcl-2 expression and the mTOR pathway. J Agric Food Chem. 2009; 57:8266–8273.
Article21. Mumoli N, Cei M. Licorice-induced hypokalemia. Int J Cardiol. 2008; 124:e42–e44.
Article22. Sontia B, Mooney J, Gaudet L, Touyz RM. Pseudohyperaldosteronism, liquorice, and hypertension. J Clin Hypertens (Greenwich). 2008; 10:153–157.
Article23. Choi HJ, Seon MR, Lim SS, Kim JS, Chun HS, Park JH. Hexane/ethanol extract of Glycyrrhiza uralensis licorice suppresses doxorubicin-induced apoptosis in H9c2 rat cardiac myoblasts. Exp Biol Med (Maywood). 2008; 233:1554–1560.
Article24. Kim JK, Oh SM, Kwon HS, Oh YS, Lim SS, Shin HK. Anti-inflammatory effect of roasted licorice extracts on lipopolysaccharide-induced inflammatory responses in murine macrophages. Biochem Biophys Res Commun. 2006; 345:1215–1223.
Article25. Li J, Lee YS, Choi JS, Sung HY, Kim JK, Lim SS, Kang YH. Roasted licorice extracts dampen high glucose-induced mesangial hyperplasia and matrix deposition through blocking Akt activation and TGF-beta signaling. Phytomedicine. 2010; 17:800–810.
Article26. Kim YH, Shin EK, Kim DH, Lee HH, Park JH, Kim JK. Antiangiogenic effect of licochalcone A. Biochem Pharmacol. 2010; 80:1152–1159.
Article27. Lee CK, Son SH, Park KK, Park JH, Lim SS, Kim SH, Chung WY. Licochalcone A inhibits the growth of colon carcinoma and attenuates cisplatin-induced toxicity without a loss of chemotherapeutic efficacy in mice. Basic Clin Pharmacol Toxicol. 2008; 103:48–54.
Article28. Kim JK, Shin EK, Park JH, Kim YH, Park JH. Antitumor and antimetastatic effects of licochalcone A in mouse models. J Mol Med (Berl). 2010; 88:829–838.
Article29. Jo EH, Kim SH, Ra JC, Kim SR, Cho SD, Jung JW, Yang SR, Park JS, Hwang JW, Aruoma OI, Kim TY, Lee YS, Kang KS. Chemopreventive properties of the ethanol extract of chinese licorice (Glycyrrhiza uralensis) root: induction of apoptosis and G1 cell cycle arrest in MCF-7 human breast cancer cells. Cancer Lett. 2005; 230:239–247.
Article30. Jo EH, Hong HD, Ahn NC, Jung JW, Yang SR, Park JS, Kim SH, Lee YS, Kang KS. Modulations of the Bcl-2/Bax family were involved in the chemopreventive effects of licorice root (Glycyrrhiza uralensis Fisch) in MCF-7 human breast cancer cell. J Agric Food Chem. 2004; 52:1715–1719.
Article31. Seon MR, Park SY, Kwon SJ, Lim SS, Choi HJ, Park H, Lim do Y, Kim JS, Lee CH, Kim J, Park JH. Hexane/ethanol extract of Glycyrrhiza uralensis and its active compound isoangustone A induce G1 cycle arrest in DU145 human prostate and 4T1 murine mammary cancer cells. J Nutr Biochem. 2012; 23:85–92.
Article32. Rafi MM, Vastano BC, Zhu N, Ho CT, Ghai G, Rosen RT, Gallo MA, DiPaola RS. Novel polyphenol molecule isolated from licorice root (Glycrrhiza glabra) induces apoptosis, G2/M cell cycle arrest, and Bcl-2 phosphorylation in tumor cell lines. J Agric Food Chem. 2002; 50:677–684.
Article33. Szliszka E, Czuba ZP, Mazur B, Sedek L, Paradysz A, Krol W. Chalcones enhance TRAIL-induced apoptosis in prostate cancer cells. Int J Mol Sci. 2009; 11:1–13.
Article34. Fu Y, Hsieh TC, Guo J, Kunicki J, Lee MY, Darzynkiewicz Z, Wu JM. Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells. Biochem Biophys Res Commun. 2004; 322:263–270.
Article35. Martinou JC, Green DR. Breaking the mitochondrial barrier. Nat Rev Mol Cell Biol. 2001; 2:63–67.
Article36. Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998; 281:1309–1312.
Article37. Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999; 15:269–290.
Article38. Plati J, Bucur O, Khosravi-Far R. Apoptotic cell signaling in cancer progression and therapy. Integr Biol (Camb). 2011; 3:279–296.
Article39. Ho PK, Hawkins CJ. Mammalian initiator apoptotic caspases. FEBS J. 2005; 272:5436–5453.
Article40. Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999; 13:1899–1911.
Article41. Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011; 30:87.
Article42. Lee SK, Park KK, Park JH, Lim SS, Chung WY. The inhibitory effect of roasted licorice extract on human metastatic breast cancer cell-induced bone destruction. Phytother Res. 2013; 27:1776–1783.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Licochalcone D Inhibits Skin Epidermal Cells Transformation through the Regulation of AKT Signaling Pathways
- The Indigenization of Licorice and Its Meaning During the Early Days of the Joseon Dynasty
- Development and Optimization of Culture Medium for the Production of Glabridin by Aspergillus eucalypticola: An Endophytic Fungus Isolated from Glycyrrhiza glabra L. (Fabaceae)
- Antagonism of licorice on selectin-mediated eodinophil and neutrophil adhesion
- Licorice-nduced Hypokalemia and Myopathy