Nutr Res Pract.
2009 Jun;3(2):156-161.
Antioxidant effect of garlic and aged black garlic in animal model of type 2 diabetes mellitus
- Affiliations
-
- 1School of Food and Life Science, Biohealth Product Research Center, Institute for Food Sciences, Inje University, 607 Obang-dong, Gimhae, Gyeongnam 621-749, Korea. fdsnkiji@inje.ac.kr
- 2Department of Hotel Culinary Arts & Bakery, Gyeongnam Provincial Namhae College, 195 Nambyon-ri, Namhae-eup, Namhae-gun, Gyeongnam 668-801, Korea.
Abstract
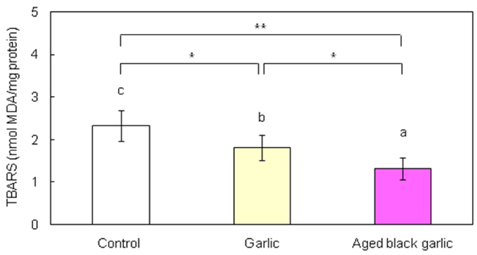
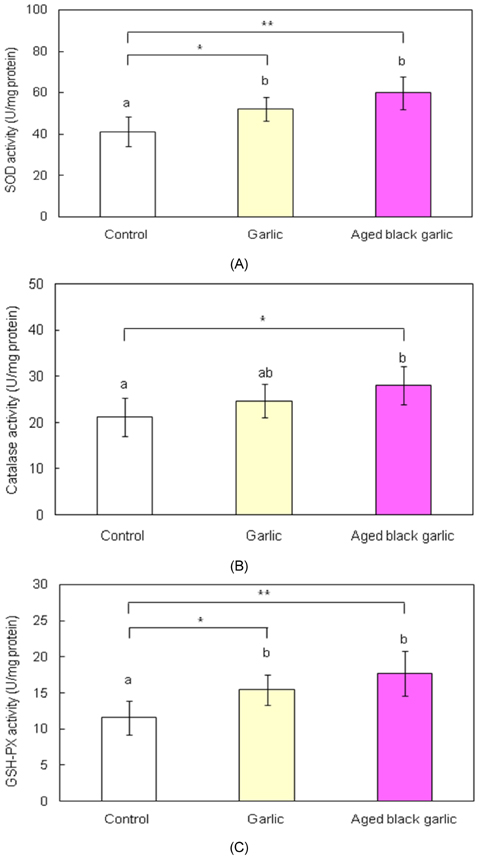
- Hyperglycemia in the diabetic state increases oxidative stress and antioxidant therapy can be strongly correlated with decreased risks for diabetic complications. The purpose of this study is to determine antioxidant effect of garlic and aged black garlic in animal model of type 2 diabetes. The antioxidant activity of garlic and aged black garlic was measured as the activity in scavenging free radicals by the trolox equivalent antioxidant capacity (TEAC) assay. Three week-old db/db mice were fed AIN-93G diet or diet containing 5% freeze-dried garlic or aged black garlic for 7 weeks after 1 week of adaptation. Hepatic levels of lipid peroxides and activities of antioxidant enzymes were measured. TEAC values of garlic and aged black garlic were 13.3 +/- 0.5 and 59.2 +/- 0.8 micromol/g wet weight, respectively. Consumption of aged black garlic significantly decreased hepatic thiobarbituric acid reactive substances (TBARS) level compared with the garlic group which showed lower TBARS level than control group (p<0.05). Activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) of garlic and aged black garlic group were significantly elevated compared to the control group. Catalase (CAT) activity of aged black garlic group was increased compared with the control group. These results show that aged black garlic exerts stronger antioxidant activity than garlic in vitro and in vivo, suggesting garlic and aged black garlic, to a greater extent, could be useful in preventing diabetic complications.
Keyword
MeSH Terms
-
Aged
Animals
Antioxidants
Catalase
Chromans
Diabetes Complications
Diabetes Mellitus
Diabetes Mellitus, Type 2
Diet
Free Radicals
Garlic
Glutathione Peroxidase
Humans
Hyperglycemia
Lipid Peroxides
Mice
Models, Animal
Oxidative Stress
Superoxide Dismutase
Thiobarbiturates
Thiobarbituric Acid Reactive Substances
Antioxidants
Catalase
Chromans
Free Radicals
Glutathione Peroxidase
Lipid Peroxides
Superoxide Dismutase
Thiobarbiturates
Thiobarbituric Acid Reactive Substances
Figure
Reference
-
1. Abei H. Catalase in the Method of Enzymatic Analysis. 1974. vol. 2. New York. USA: Academic Press;673–684.2. Ahmad MS, Pischetsrieder M, Ahmed A. Aged garlic extract and S-allyl cysteine prevent formation of advanced glycation endproducts. Eur J Pharmacol. 2007. 561:32–38.
Article3. Al-Qattan K, Thomson M, Ali M. Garlic (Allium sativum) and ginger (Zingiber officinale) attenuate structural nephropathy progression in streptozotocin-induced diabetic rats. The European e-Journal of Clinical Nutrition and Metabolism. 2008. 3:e62–e71.
Article4. Amagase H. Clarjfying the real bioactive constituents of garlic. J Nutr. 2006. 136:716S–725S.5. Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001. 131:955S–962S.
Article6. American Diabetes Association. Management of dyslipidemia in adults with diabetes (Position Statement). Diabetes Care. 1999. 22:56–59.7. Banerjee SK, Mukherjee PK, Maulik SK. Garlic as an antioxidant: the good, the bad and the ugly. Phytother Res. 2003. 17:97–106.
Article8. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976. 72:248–254.
Article9. Diabetes Surveillance Report. Centers for Disease Control and Prevention. 1999. Accessed on 4/07/2009. Atlanta, GA: US Department of Health and Human Services;http://www.cdc.gov.10. Ceriello A. Oxidative stress and diabetes-associated complications. Endocr Pract. 2006. 12:60–62.
Article11. Cheng AYY, Josse RG. Intestinal absorption inhibitors for type 2 diabetes mellitus: prevention and treatment. Drug Discovery Today: Therapeutic Strategies. 2004. 1:201–206.
Article12. Corzo-Martinez M, Corso N, Villamiel M. Biological properties of onions and garlic. Trends in Food Science, Technology. 2007. 18:609–625.
Article13. Drobiova H, Thomson M, Al-Qattan K, Peltonen-Shalaby R, Al-Amin Z, Ali M. Garlic increases antioxidant levels in diabetic and hypertensive rats determined by a modified peroxidase Method. Evid Based Complement Alternat Med. 2009. eCAM:1–7.
Article14. Eidi A, Eidi M, Esmaeili E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine. 2006. 13:624–629.
Article15. Fujita H, Yamagami T, Ohshima K. Long-term ingestion of a fermented soybean-derived Touchi-extract with alpha-glucosidase inhibitory activity is safe and effective in humans with borderline and mild type-2 diabetes. J Nutr. 2001. 131:2105–2108.
Article16. Haron D. The aging: major risk factor for disease and death. Proc Natl Acad Sci U S A. 1991. 88:5360–5364.17. Imai J, Ide N, Nagae S, Moriguchi T, Matsuura H, Itakura Y. Antioxidant and radical scavenging effects of aged garlic extract and its constituents. Planta Med. 1994. 60:417–420.
Article18. Jang EK, Seo JH, Lee SP. Physiological activity and antioxidative effects of aged black garlic (Allium sativum L.) extract. Korean Society of Food Science and Technology. 2008. 40:443–448.19. Jung UJ, Lee MK, Park YB, Jeon SM, Choi MS. Antihyperglycemic and antioxidant properties of caffeic acid in db/db mice. J Pharmacol Exp Ther. 2006. 318:476–483.
Article20. Kaneto H, Nakatani Y, Kawamori D, Miyatsuka T, Matsuoka TA, Matsuhisa M, Yamasaki Y. Role of oxidative stress, endoplasmic reticulum stress, and c-Jun N-terminal kinase in pancreatic β-cell dysfunction and insulin resistance. Int J Biochem Cell Biol. 2005. 37:1595–1608.
Article21. Kang MJ, Lee SJ, Shin JH, Kang SK, Kim JG, Sung NJ. Effect of garlic with different processing on lipid metabolism in 1% cholesterol fed rats. Journal of the Korean Society of Food Science and Nutrition. 2008. 37:162–169.
Article22. Kang YH, Park YK, Lee GD. The nitrite scavenging and electron donating ability of phenolic compounds. Korean Society of Food Science and Technology. 1996. 28:232–239.23. Kanth RV, Reddy PUM, Raju TN. Attenuation of streptozotocin-induced oxidative stress in hepatic and intestinal tissues of Wistar rat by methanolic-garlic extract. Acta Diabetol. 2008. 45:243–251.
Article24. King H, Aubert RE, Herman WH. Global burden of diabetes, 1995-2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998. 21:1414–1431.
Article25. Lean ME, Noroozi M, Kelly I, Burns J, Talwar D, Sattar N, Crozier A. Dietary flavonols protect diabetic human lymphocytes against oxidative damage to DNA. Diabetes. 1999. 48:176–181.
Article26. Maritim AC, Sanders RA, Watkins JB 3rd. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003. 17:24–38.
Article27. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974. 47:469–474.
Article28. Ohkawa H, Ohishi N, Yake K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979. 95:351–358.
Article29. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967. 70:158–169.30. Park SA, Choi MS, Jung UJ, Kim MJ, Kim DJ, Park HM, Park YB, Lee MK. Eucommia ulmoides Oliver Leaf extract increases endogenous antioxidant activity in type 2 diabetic mice. J Med Food. 2006. 9:474–479.
Article31. Queiroz YS, Ishimoto EY, Bastos DH, Sampaio GR, Torres EA. Garlic (Allium sativum L.) and ready-to-eat garlic products: In vitro antioxidant activity. Food Chem. 2009. 115:371–374.
Article32. Rahimi R, Nikfar S, Larijani B, Abdollahi M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed Pharmacother. 2005. 59:365–373.
Article33. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-evans C. Antioxidant activity applying an improved abts radical cation decolorization assay. Free Radic Biol Med. 1999. 26:1231–1237.
Article34. Seo KI, Choi MS, Jung UJ, Kim HJ, Yeo J, Jeon SM, Lee MK. Effect of curcumin supplementation on blood glucose, plasma insulin, and glucose homeostasis related enzyme activities in diabetic db/db mice. Mol Nutr Food Res. 2008. 52:995–1004.
Article35. Seo YJ, Gweon OC, Lee YM, Kang MJ, Kim JI. Effect of garlic and aged black garlic on hyperglycemia and dyslipidemia in animal model of type 2 diabetes mellitus. Journal of Food Science and Nutrition. 2009. 14:1–7.
Article36. Shin CH, Ihm J. Effects of S-allycysteine on oxidative stress in streptozotocin-induced diabetic rats. Journal of Korean Society of Endocrinology. 2008. 23:129–136.
Article37. Sinclair AJ, Girling AJ, Gray L, Lunec J, Barnett AH. An investigation of the relationship between free radical activity and vitamin C metabolism in elderly diabetic subjects with retinopathy. Gerontology. 1992. 38:268–274.
Article38. The Diabetes Control and Complications Trial (DCCT) Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in the diabetes control in insulin-dependent diabetes mellitus. N Engl J Med. 1993. 329:977–986.39. UK Prospective Diabetes Study (UKPDS) Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes. UKPDS 38. BMJ. 1998. 317:703–713.40. Youn JY, Park HY, Cho KH. Anti-hyperglycemic activity of Commelina communis L.: inhibition of α-glucosidase. Diabetes Res Clin Pract. 2004. 66S:S149–S155.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antioxidant mechanism of black garlic extract involving nuclear factor erythroid 2-like factor 2 pathway
- Two Cases of Irritant Contact Dermatitis due to Garlic
- A study on the effect of garlic to the heavy metal poisoning of rat
- Antioxidant Effect of Garlic Supplement Against Exercise-Induced Oxidative Stress in Rats
- A Case of Irritant Contact Dermatitis due to Garlic