Nutr Res Pract.
2017 Jun;11(3):206-213. 10.4162/nrp.2017.11.3.206.
Antioxidant mechanism of black garlic extract involving nuclear factor erythroid 2-like factor 2 pathway
- Affiliations
-
- 1Department of Food Science and Nutrition, 119, Dandae-ro, Dongnam-gu, Cheonan-si, Chungnam 31116, Korea. wkkim@dankook.ac.kr
- KMID: 2407768
- DOI: http://doi.org/10.4162/nrp.2017.11.3.206
Abstract
- BACKGROUN/OBJECTIVES: Although studies have revealed that black garlic is a potent antioxidant, its antioxidant mechanism remains unclear. The objective of this study was to determine black garlic's antioxidant activities and possible antioxidant mechanisms related to nuclear factor erythroid 2-like factor 2 (Nrf2)-Keap1 complex.
METHODS
/MATERIALS: After four weeks of feeding rats with a normal fat diet (NF), a high-fat diet (HF), a high-fat diet with 0.5% black garlic extract (HF+BGE 0.5), a high-fat diet with 1.0% black garlic extract (HF+BGE 1.0), or a high-fat diet with 1.5% black garlic extract (HF+BGE 1.5), plasma concentrations of glucose, insulin,homeostatic model assessment of insulin resistance (HOMA-IR) were determined. As oxidative stress indices, plasma concentrations of thiobarbituric acid reactive substances (TBARS) and 8-isoprostaglandin F2α (8-iso-PGF) were determined. To measure antioxidant capacities, plasma total antioxidant capacity (TAC) and activities of antioxidant enzymes in plasma and liver were determined. The mRNA expression levels of antioxidant related proteins such as Nrf2, NAD(P)H: quinone-oxidoreductase-1 (NQO1), heme oxygenase-1 (HO-1), glutathione reductase (GR), and glutathione S-transferase alpha 2 (GSTA2) were examined.
RESULTS
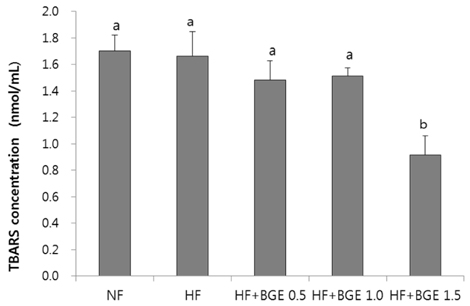
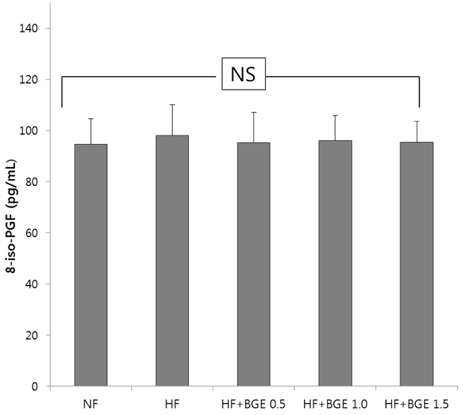
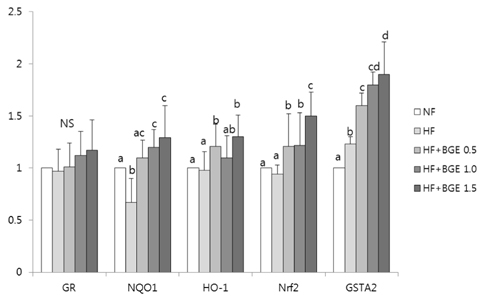
Plasma glucose level, plasma insulin level, and HOMA-IR in black garlic supplemented groups were significantly (P < 0.05) lower than those in the HF group without dose-dependent effect. Plasma TBARS concentration and TAC in the HF+BGE 1.5 group were significantly decreased compared to those of the HF group. The activities of catalase and glutathione peroxidase were significantly (P < 0.05) increased in the HF+BGE 1.0 and HF+BGE 1.5 groups compared to those of the HF group. The mRNA expression levels of hepatic Nrf2, NQO1, HO-1, and GSTA2 were significantly (P < 0.05) increased in the HF with BGE groups compared to those in the HF group.
CONCLUSIONS
The improvements of blood glucose homeostasis and antioxidant systems in rats fed with black garlic extract were related to mRNA expression levels of Nrf2 related genes.
Keyword
MeSH Terms
-
Animals
Blood Glucose
Catalase
Diet
Diet, High-Fat
Garlic*
Glucose
Glutathione Peroxidase
Glutathione Reductase
Glutathione Transferase
Heme Oxygenase-1
Homeostasis
Insulin
Insulin Resistance
Liver
Oxidative Stress
Plasma
Rats
RNA, Messenger
Thiobarbituric Acid Reactive Substances
Blood Glucose
Catalase
Glucose
Glutathione Peroxidase
Glutathione Reductase
Glutathione Transferase
Heme Oxygenase-1
Insulin
RNA, Messenger
Thiobarbituric Acid Reactive Substances
Figure
Cited by 1 articles
-
Erratum: Antioxidant mechanism of black garlic extract involving nuclear factor erythroid 2-like factor 2 pathway
Ae Wha Ha, Woo Kyoung Kim
Nutr Res Pract. 2017;11(4):347-347. doi: 10.4162/nrp.2017.11.4.347.
Reference
-
1. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004; 114:1752–1761.
Article2. Lee OH, Kwon YI, Hong HD, Park CS, Lee BY, Kim YC. Production of reactive oxygen species and changes in antioxidant enzyme activities during differentiation of 3T3-L1 adipocyte. J Korean Soc Appl Biol Chem. 2009; 52:70–75.
Article3. Banerjee SK, Mukherjee PK, Maulik SK. Garlic as an antioxidant: the good, the bad and the ugly. Phytother Res. 2003; 17:97–106.
Article4. Lee YM, Gweon OC, Seo YJ, Im J, Kang MJ, Kim MJ, Kim JI. Antioxidant effect of garlic and aged black garlic in animal model of type 2 diabetes mellitus. Nutr Res Pract. 2009; 3:156–161.
Article5. Kim MH, Kim MJ, Lee JH, Han JI, Kim JH, Sok DE, Kim MR. Hepatoprotective effect of aged black garlic on chronic alcohol-induced liver injury in rats. J Med Food. 2011; 14:732–738.
Article6. Jang EK, Seo JH, Lee SP. Physiological activity and antioxidative effects of aged black garlic (Allium sativum L.) extract. Korean J Food Sci Technol. 2008; 40:443–448.7. Wang Q, Wang XL, Liu HR, Rose P, Zhu YZ. Protective effects of cysteine analogues on acute myocardial ischemia: novel modulators of endogenous H(2)S production. Antioxid Redox Signal. 2010; 12:1155–1165.
Article8. Bełtowski J. Hydrogen sulfide in pharmacology and medicine--an update. Pharmacol Rep. 2015; 67:647–658.9. Bhuiyan AI, Papajani VT, Paci M, Melino S. Glutathione-garlic sulfur conjugates: slow hydrogen sulfide releasing agents for therapeutic applications. Molecules. 2015; 20:1731–1750.
Article10. Amagase H. Clarifying the real bioactive constituents of garlic. J Nutr. 2006; 136:716S–725S.
Article11. Toledano-Medina MA, Pérez-Aparicio J, Moreno-Rojas R, Merinas-Amo T. Evolution of some physicochemical and antioxidant properties of black garlic whole bulbs and peeled cloves. Food Chem. 2016; 199:135–139.
Article12. Shi H, Jing X, Wei X, Perez RG, Ren M, Zhang X, Lou H. S-allyl cysteine activates the Nrf2-dependent antioxidant response and protects neurons against ischemic injury in vitro and in vivo. J Neurochem. 2015; 133:298–308.
Article13. Jiménez-Osorio AS, González-Reyes S, Pedraza-Chaverri J. Natural Nrf2 activators in diabetes. Clin Chim Acta. 2015; 448:182–192.
Article14. Ha AW, Ying T, Kim WK. The effects of black garlic (Allium satvium) extracts on lipid metabolism in rats fed a high fat diet. Nutr Res Pract. 2015; 9:30–36.
Article15. Cacho J, Sevillano J, de Castro J, Herrera E, Ramos MP. Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague-Dawley rats. Am J Physiol Endocrinol Metab. 2008; 295:E1269–E1276.
Article16. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–419.
Article17. Santos MT, Valles J, Aznar J, Vilches J. Determination of plasma malondialdehyde-like material and its clinical application in stroke patients. J Clin Pathol. 1980; 33:973–976.
Article18. Rice-Evans CA. Measurement of total antioxidant activity as a marker of antioxidant status in vivo: procedures and limitations. Free Radic Res. 2000; 33:Suppl. S59–S66.19. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497–509.
Article20. Ha AW, Na SJ, Kim WK. Antioxidant effects of fucoxanthin rich powder in rats fed with high fat diet. Nutr Res Pract. 2013; 7:475–480.
Article21. Aebi H. Catalase in vitro. Methods Enzymol. 1984; 105:121–126.22. Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol. 1983; 23:239–257.
Article23. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967; 70:158–169.24. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193:265–275.
Article25. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔ C(T)) method. Methods. 2001; 25:402–408.
Article26. Wang YP, Cheng ML, Zhang BF, Mu M, Wu J. Effects of blueberry on hepatic fibrosis and transcription factor Nrf2 in rats. World J Gastroenterol. 2010; 16:2657–2663.
Article27. Shin JH, Lee CW, Oh SJ, Yun J, Kang MR, Han SB, Park H, Jung JC, Chung YH, Kang JS. Hepatoprotective effect of aged black garlic extract in rodents. Toxicol Res. 2014; 30:49–54.
Article28. Jung YM, Lee SH, Lee DS, You MJ, Chung IK, Cheon WH, Kwon YS, Lee YJ, Ku SK. Fermented garlic protects diabetic, obese mice when fed a high-fat diet by antioxidant effects. Nutr Res. 2011; 31:387–396.
Article29. Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015; 6:456–480.
Article30. Seo YJ, Gweon OC, Im J, Lee YM, Kang MJ, Kim JI. Effect of garlic and aged black garlic on hyperglycemia and dyslipidemia in animal model of type 2 diabetes mellitus. J Food Sci Nutr. 2009; 14:1–7.
Article31. Eidi A, Eidi M, Esmaeili E. Antidiabetic effect of garlic (Allium sativum L.) in normal and streptozotocin-induced diabetic rats. Phytomedicine. 2006; 13:624–629.
Article32. Jeong YY, Ryu JH, Shin JH, Kang MJ, Kang JR, Han J, Kang D. Comparison of anti-oxidant and anti-inflammatory effects between fresh and aged black garlic extracts. Molecules. 2016; 21:430.
Article33. Bae SE, Cho SY, Won YD, Lee SH, Park HJ. Changes in S-allyl cysteine contents and physicochemical properties of black garlic during heat treatment. Lebenson Wiss Technol. 2014; 55:397–402.
Article34. Yang CS, Chhabra SK, Hong JY, Smith TJ. Mechanisms of inhibition of chemical toxicity and carcinogenesis by diallyl sulfide (DAS) and related compounds from garlic. J Nutr. 2001; 131:1041S–1045S.
Article35. Patrono C, Falco A, Davì G. Isoprostane formation and inhibition in atherothrombosis. Curr Opin Pharmacol. 2005; 5:198–203.
Article36. Uruno A, Furusawa Y, Yagishita Y, Fukutomi T, Muramatsu H, Negishi T, Sugawara A, Kensler TW, Yamamoto M. The Keap1-Nrf2 system prevents onset of diabetes mellitus. Mol Cell Biol. 2013; 33:2996–3010.
Article37. Zheng H, Whitman SA, Wu W, Wondrak GT, Wong PK, Fang D, Zhang DD. Therapeutic potential of Nrf2 activators in streptozotocin-induced diabetic nephropathy. Diabetes. 2011; 60:3055–3066.
Article38. Jiménez-Osorio AS, Picazo A, González-Reyes S, Barrera-Oviedo D, Rodríguez-Arellano ME, Pedraza-Chaverri J. Nrf2 and redox status in prediabetic and diabetic patients. Int J Mol Sci. 2014; 15:20290–20305.
Article39. Baluchnejadmojarad T, Kiasalari Z, Afshin-Majd S, Ghasemi Z, Roghani M. S-allyl cysteine ameliorates cognitive deficits in streptozotocin-diabetic rats via suppression of oxidative stress, inflammation, and acetylcholinesterase. Eur J Pharmacol. 2017; 794:69–76.
Article40. Zhu YF, Li XH, Yuan ZP, Li CY, Tian RB, Jia W, Xiao ZP. Allicin improves endoplasmic reticulum stress-related cognitive deficits via PERK/Nrf2 antioxidative signaling pathway. Eur J Pharmacol. 2015; 762:239–246.
Article41. Li XH, Li CY, Xiang ZG, Hu JJ, Lu JM, Tian RB, Jia W. Allicin ameliorates cardiac hypertrophy and fibrosis through enhancing of Nrf2 antioxidant signaling pathways. Cardiovasc Drugs Ther. 2012; 26:457–465.
Article42. Guyonnet D, Belloir C, Suschetet M, Siess MH, Le Bon AM. Antimutagenic activity of organosulfur compounds from Allium is associated with phase II enzyme induction. Mutat Res. 2001; 495:135–145.
Article43. Lee IC, Kim SH, Baek HS, Moon C, Kang SS, Kim SH, Kim YB, Shin IS, Kim JC. The involvement of Nrf2 in the protective effects of diallyl disulfide on carbon tetrachloride-induced hepatic oxidative damage and inflammatory response in rats. Food Chem Toxicol. 2014; 63:174–185.
Article44. Tobón-Velasco JC, Vázquez-Victorio G, Macías-Silva M, Cuevas E, Ali SF, Maldonado PD, González-Trujano ME, Cuadrado A, Pedraza-Chaverrí J, Santamaría A. S-allyl cysteine protects against 6-hydroxydopamine-induced neurotoxicity in the rat striatum: involvement of Nrf2 transcription factor activation and modulation of signaling kinase cascades. Free Radic Biol Med. 2012; 53:1024–1040.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Antioxidant mechanism of black garlic extract involving nuclear factor erythroid 2-like factor 2 pathway
- Role of Nuclear Factor Erythroid 2-Related Factor 2 in Chronic Obstructive Pulmonary Disease
- Antioxidant and anti-inflammatory effects and mechanism of Abeliophyllum distichum leaf extract in RAW264.7 macrophages
- Antioxidant effect of garlic and aged black garlic in animal model of type 2 diabetes mellitus
- Kelch-like ECH-associated Protein 1/Nuclear Factor Erythroid 2-related Factor 2 Pathway and Its Interplay with Oncogenes in Lung Tumorigenesis