J Clin Neurol.
2005 Apr;1(1):81-91. 10.3988/jcn.2005.1.1.81.
Comparison of the Protective Effect of Indole beta-carbolines and R-(-)-deprenyl Against Nitrogen Species-Induced Cell Death in Experimental Culture Model of Parkinson's Disease
- Affiliations
-
- 1Department of Neurology, Seoul Veterans Hospital, Seoul, Korea. kimdeung@hanmail.net
- 2Department of Pharmacology, Chung-Ang University College of Medicine, Seoul, Korea. leecs@cau.ac.kr
- KMID: 2287717
- DOI: http://doi.org/10.3988/jcn.2005.1.1.81
Abstract
- BACKGROUND
The membrane permeability transition of mitochondria has been suggested to be involved in toxic and oxidative forms of cell injury. Mitochondrial dysfunction is considered to play a critical role in neurodegeneration in Parkinson's disease. Despite the suggestion that indole beta-carbolines may be neurotoxic, these compounds provide a protective effect against cytotoxicity of other neurotoxins. In addition, the effect of indole beta-carbolines on change in the mitochondrial membrane permeability due to reactive nitrogen species (RNS), which may lead to cell death, has not been clarified.
METHODS
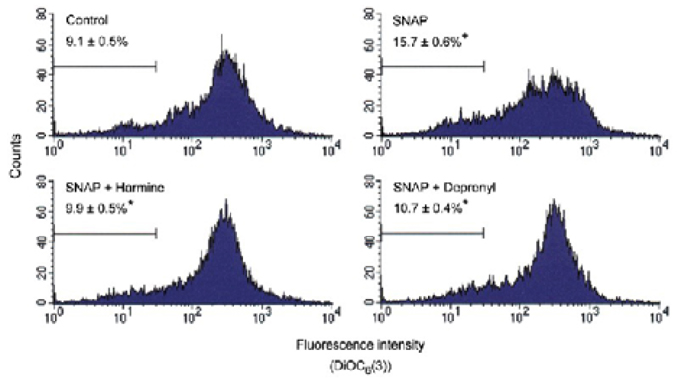
Differentiated PC12 cells were used as the experimental culture model for the investigation of neuronal cell injury, which occurs in Parkinson's disease. The effect of indole beta-carbolines (harmalol and harmine) on differentiated PC12 cells against toxicity of S-nitroso-N-acetyl-DL-penicillamine (SNAP) was determined by measuring the effect on the change in transmembrane potential, cytochrome c release, formation of ROS, GSH contents, caspase-3 activity and cell viability, and was compared to that of R-(-)-deprenyl.
RESULTS
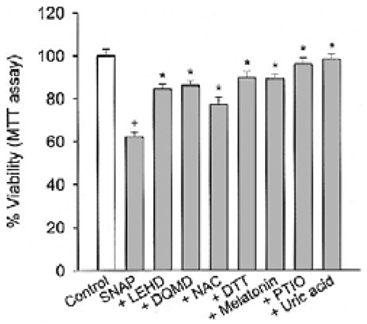
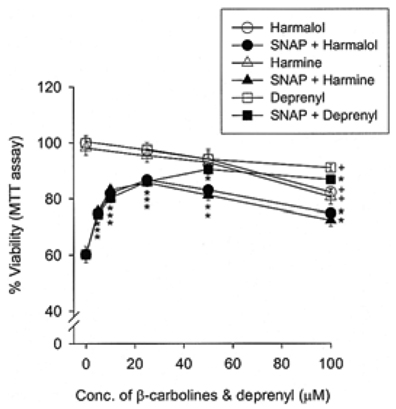
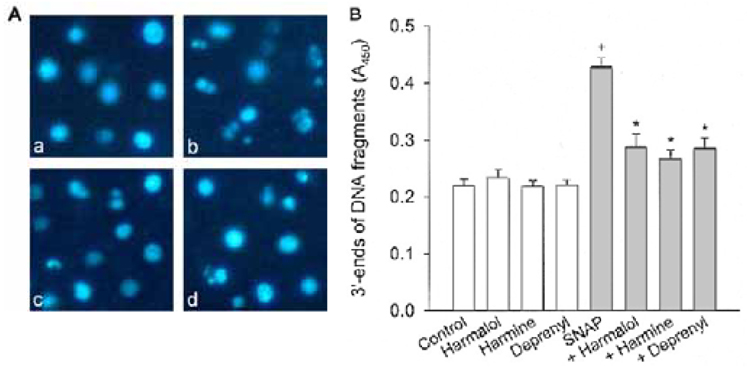
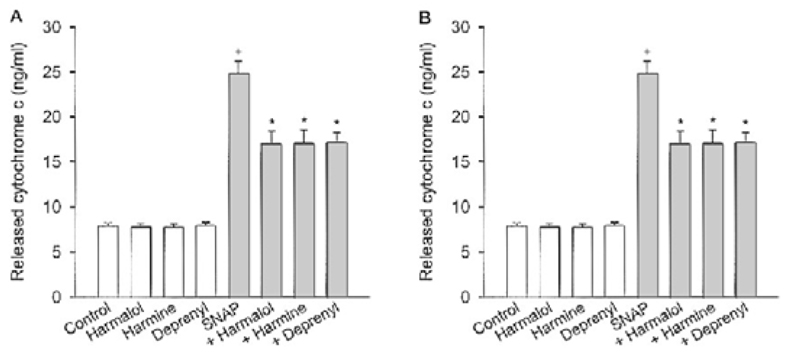
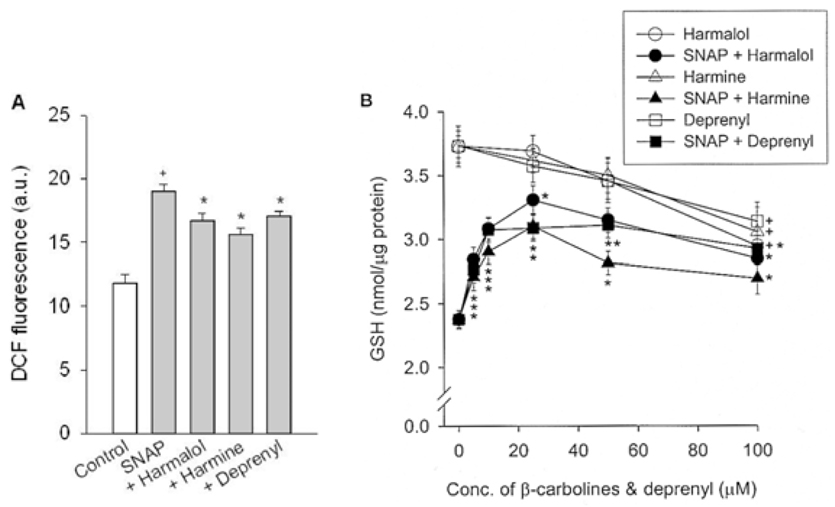
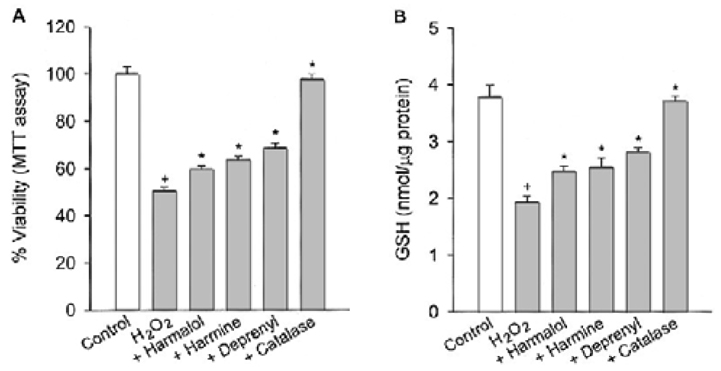
Specific inhibitors of caspases (z-LEHD.fmk, z-DQMD.fmk) and antioxidants (N-acetylcysteine, dithiothreitol, melatonin, carboxy-PTIO and uric acid) depressed cell death in PC12 cells due to SNAP. beta-Carbolines and R-(-)-deprenyl attenuated the SNAP-induced cell death and GSH depletion concentration dependently with a maximal inhibitory effect at 25-50 microM. The compounds inhibited the nuclear damage, decrease in mitochondrial transmembrane potential, cytochrome c release and formation of reactive oxygen species caused by SNAP in PC12 cells. beta-Carbolines and R-(-)-deprenyl attenuated the H2O2-induced cell death and depletion of GSH.
CONCLUSIONS
The results suggest that indole beta-carbolines attenuate the SNAP-induced viability loss in PC12 cells by inhibition of change in the mitochondrial membrane permeability, which may be caused by free radicals. Indole beta-carbolines appear to exert a protective effect against the nitrogen species-mediated neuronal cell injury in Parkinson's disease comparable to R-(-)-deprenyl.
Keyword
MeSH Terms
-
Animals
Antioxidants
Carbolines*
Caspase 3
Caspases
Cell Death*
Cell Survival
Cytochromes c
Dithiothreitol
Free Radicals
Melatonin
Membrane Potentials
Membranes
Mitochondria
Mitochondrial Membranes
Neurons
Neurotoxins
Nitrogen*
Parkinson Disease*
PC12 Cells
Permeability
Reactive Nitrogen Species
Reactive Oxygen Species
Antioxidants
Carbolines
Caspase 3
Caspases
Cytochromes c
Dithiothreitol
Free Radicals
Melatonin
Neurotoxins
Nitrogen
Reactive Nitrogen Species
Reactive Oxygen Species
Figure
Reference
-
1. Chandra J, Samali A, Orrenius S. Triggering and modulation of apoptosis by oxidative stress. Free Radic Biol Med. 2000. 29:323–333.
Article2. Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol. 2003. 53:suppl 3. S26–S38.
Article3. Lee CS, Song EH, Park SY, Han ES. Combined effect of dopamine and MPP+ on membrane permeability in mitochondria and cell viability in PC12 cells. Neurochem Int. 2003. 43:147–154.
Article4. Hunot S, Boissiere F, Faucheux B, Brugg B, Mouatt-Prigent A, Agid Y, et al. Nitric oxide synthase and neuronal vulnerability in Parkinson's disease. Neuroscience. 1996. 72:355–363.
Article5. Good PF, Hsu A, Wener P, Perl DP, Olanow CW. Protein nitration in Parkinson's disease. J Neuropathol Exp Neurol. 1998. 57:338–342.
Article6. Hantraye P, Brouillet E, Ferrante R, Palfi S, Dolan R, Matthews RT, et al. Inhibition of neuronal nitric oxide synthase prevents MPTP-induced parkinsonism in baboons. Nat Med. 1996. 2:1017–1021.
Article7. Pryor WA, Squadrito GL. The chemistry of peroxynitrite: a product from the reaction of nitric oxide and superoxide. Am J Physiol. 1995. 268:L699–L722.8. Stewart VC, Heales SJR. Nitric oxide-induced mitochondrial dysfunction: implications for neurodegeneration. Free Radic Biol Med. 2003. 34:287–303.
Article9. Ghafourifar P, Schenk U, Klein SD, Richter C. Mitochondrial nitric-oxide synthase stimulation causes cytochrome c release from isolated mitochondria. Evidence for intramitochondrial peroxynitrite formation. J Biol Chem. 1999. 274:31185–31188.
Article10. Bal-Price A, Brown GC. Nitric-oxide-induced necrosis and apoptosis in PC12 cells mediated by mitochondria. J Neurochem. 2000. 75:1455–1464.
Article11. Kadota T, Yamaai T, Saito Y, Akita Y, Kawashima S, Moroi K, et al. Expression of dopamine transporter at the tips of growing neurites of PC12 cells. J Histochem Cytochem. 1996. 44:989–996.
Article12. Birkmayer W, Knoll J, Riederer P, Youdim MB, Hars V, Marton J. Increased life expectancy resulting from addition of L-deprenyl to Madopar treatment in Parkinson's disease: a longterm study. J Neural Transm. 1985. 64:113–127.
Article13. Tatton WG, Chalmers-Redman RM. Modulation of gene expression rather than monoamine oxidase inhibition: (-)-deprenyl-related compounds in controlling neurodegeneration. Neurology. 1996. 47:S171–S183.
Article14. Wu RM, Chen RC, Chiueh CC. Effect of MAO-B inhibitors on MPP+ toxicity in Vivo. Ann N Y Acad Sci. 2000. 899:255–261.15. Albores R, Neafsey EJ, Drucker G, Fields JZ, Collins MA. Mitochondrial respiratory inhibition by N-methylated β-carboline derivatives structurally resembling N-methyl-4-phenylpyridine. Proc Natl Acad Sci USA. 1990. 87:9368–9372.
Article16. Gearhart DA, Toole PF, Beach JW. Identification of brain proteins that interact with 2-methylnorharman. An analog of the parkinsonian-inducing toxin, MPP+. Neurosci Res. 2002. 44:255–265.17. Cobuzzi RJ Jr, Neafsey EJ, Collins MA. Differential cytotoxicities of N-methyl-β-carbolinium analogues of MPP+ in PC12 cells: insights into potential neurotoxicants in Parkinson's disease. J Neurochem. 1994. 62:1503–1510.
Article18. O'Hearn E, Molliver ME. Degeneration of Purkinje cells in parasagittal zones of the cerebellar vermis after treatment with ibogaine or harmaline. Neuroscience. 1993. 55:303–310.19. Tse SYH, Mak IT, Dickens BF. Antioxidative properties of harman and β-carboline alkaloids. Biochem Pharmacol. 1991. 42:459–464.20. Fernandez de Arriva A, Lizcano JM, Balsa MD, Unzeta M. Inhibition of monoamine oxidase from bovine retina by β-carbolines. J Pharm Pharmacol. 1994. 46:809–813.
Article21. Maher P, Davis JB. The role of monoamine metabolism in oxidative glutamate toxicity. J Neurosci. 1996. 16:6394–6401.
Article22. Kim DH, Jang YY, Han ES, Lee CS. Protective effect of harmaline and harmalol against dopamine- and 6-hydroxydopamine-induced oxidative damage of brain mitochondria and synaptosomes, and viability loss of PC12 cells. Eur J Neurosci. 2001. 13:1861–1872.
Article23. Tatton WG, Chalmers-Redman RME, Ju WJH, Mammen M, Carlile GW, Pong AW, et al. Propargylamines induce antiapoptotic new protein synthesis in serum-and nerve growth factor (NGF)-withdrawn, NGF-differentiated PC-12 cells. J Pharmacol Exp Ther. 2002. 301:753–764.
Article24. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983. 65:55–63.
Article25. Oberhammer FA, Pavelka M, Sharma S, Tiefenbacher R, Purchio AF, Bursch W, et al. Induction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor β1. Proc Natl Acad Sci USA. 1992. 89:5408–5412.
Article26. Lizard G, Miguet C, Bessede G, Monier S, Gueldry S, Neel D, et al. Impairment with various antioxidants of the loss of mitochondrial transmembrane potential and of the cytosolic release of cytochrome c occurring during 7-ketocholesterol-induced apoptosis. Free Radic Biol Med. 2000. 28:743–753.
Article27. Fu W, Luo H, Parthasarathy S, Mattson MP. Catecholamines potentiate amyloid β-peptide neurotoxicity: involvement of oxidative stress, mitochondrial dysfunction, and perturbed calcium homeostasis. Neurobiol Dis. 1998. 5:229–243.
Article28. van Klaveren RJ, Hoet PH, Pype JL, Demedts M, Nemery B. Increase in gamma-glutamyltransferase by glutathione depletion in rat type II pneumocytes. Free Radic Biol Med. 1997. 22:525–534.
Article29. Polster BM, Fiskum G. Mitochondrial mechanisms of neuronal cell apoptosis. J Neurochem. 2004. 90:1281–1289.30. Jurma OP, Hom DG, Andersen JK. Decreased glutathione results in calcium-mediated cell death in PC12. Free Radic Biol Med. 1997. 23:1055–1066.
Article31. Airaksinen MM, Kari I. β-Carbolines, psychoactive compounds in the mammalian body. Part I; Occurrence, origin and metabolism. Med Biol. 1981. 59:21–34.32. Beck O, Faull KF. Concentrations of the enantiomers of 5-hydroxymethtryptoline in mammalian urine: implications for in vivo biosynthesis. Biochem Pharmacol. 1986. 35:2636–2639.
Article33. Matsubara K, Kobayashi S, Kobayashi Y, Yamashita K, Koide H, Hatta M, et al. β-Carbolinium cations, endogenous MPP+ analogs, in the lumbar cerebrospinal fluid of patients with Parkinson's disease. Neurology. 1995. 45:2240–2245.
Article34. Matsubara K, Gonda T, Sawada H, Uezono T, Kobayashi Y, Kawamura T, et al. Endogenously occurring β-carboline induces parkinsonism in nonprimate animals: a possible causative protoxin in idiopathic Parkinson's disease. J Neurochem. 1998. 70:727–735.
Article35. Holmstedt B, Lingren JE. Efron DH, Holmstdet B, Kline NS, editors. Chemical constituents and pharmacology of south American snuffs. Ethnopharmacologic search for psychoactive drugs. 1967. New York: Raven Press;339–373.36. Ogawa Y, Adachi J, Tatsuno Y. Accumulation of 1-methyl-tetrahydro-β-carboline-3-carboxylic acid in blood and organs of rat. A possible causative substance of eosinophilia-myalgia syndrome associated with ingestion of L-tryptophan. Arch Toxicol. 1993. 67:290–293.
Article37. Pari K, Sundari CS, Chandani S, Balasubramanian D. β-Carbolines that accumulate in human tissues may serve a protective role against oxidative stress. J Biol Chem. 2000. 275:2455–2462.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Glucose Levels in Culture Medium Determine Cell Death Mode in MPP+-treated Dopaminergic Neuronal Cells
- Inhibitory Effect of Flavonoid Luteolin on 6-Hydroxydopamine Cytotoxicity via Suppression of Apoptosis-Related Protein Activation
- Monoamine Oxidase Inhibitors Attenuate Cytotoxicity of 1-Methyl-4-phenylpyridinium by Suppressing Mitochondrial Permeability Transition
- Protective Effect of Metformin on Gentamicin-Induced Vestibulotoxicity in Rat Primary Cell Culture
- Rhamnazin inhibits LPS-induced inflammation and ROS/RNS in raw macrophages