J Nutr Health.
2016 Oct;49(5):288-294. 10.4163/jnh.2016.49.5.288.
Rhamnazin inhibits LPS-induced inflammation and ROS/RNS in raw macrophages
- Affiliations
-
- 1Department of Dental Hygiene, Busan Women's College, Busanjin-Gu, Busan 47228, Korea. yjknutr@yahoo.co.kr
- KMID: 2357131
- DOI: http://doi.org/10.4163/jnh.2016.49.5.288
Abstract
- PURPOSE
The aim of this work was to investigate the beneficial effects of rhamnazin against inflammation, reactive oxygen species (ROS)/reactive nitrogen species (RNS), and anti-oxidative activity in murine macrophage RAW264.7 cells.
METHODS
To examine the beneficial properties of rhamnazin on inflammation, ROS/ RNS, and anti-oxidative activity in the murine macrophage RAW264.7 cell model, several key markers, including COX and 5-LO activities, NO"¢, ONOO-, total reactive species formation, lipid peroxidation, "¢Oâ‚‚ levels, and catalase activity were estimated.
RESULTS
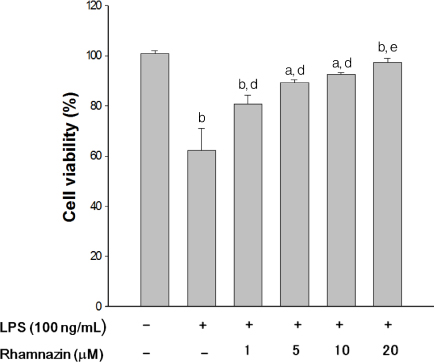
Results show that rhamnazin was protective against LPS-induced cytotoxicity in macrophage cells. The underlying action of rhamnazin might be through modulation of ROS/RNS and anti-oxidative activity through regulation of total reactive species production, lipid peroxidation, catalase activity, and "¢Oâ‚‚, NO"¢, and ONOO"¢ levels. In addition, rhamnazin down-regulated the activities of pro-inflammatory COX and 5-LO.
CONCLUSION
The plausible action by which rhamnazin renders its protective effects in macrophage cells is likely due to its capability to regulate LPS-induced inflammation, ROS/ RNS, and anti-oxidative activity.
MeSH Terms
Figure
Reference
-
1. Kim YJ, Kim YA, Yokozawa T. Attenuation of oxidative stress and inflammation by gravinol in high glucose-exposed renal tubular epithelial cells. Toxicology. 2010; 270(2-3):106–111.
Article2. Kim YJ, Kim YA, Yokozawa T. Protection against oxidative stress, inflammation, and apoptosis of high-glucose-exposed proximal tubular epithelial cells by astaxanthin. J Agric Food Chem. 2009; 57(19):8793–8797.
Article3. Kim YJ, Kim YA, Yokozawa T. Pycnogenol modulates apoptosis by suppressing oxidative stress and inflammation in high glucosetreated renal tubular cells. Food Chem Toxicol. 2011; 49(9):2196–2201.
Article4. Hofmann B, Steinhilber D. 5-Lipoxygenase inhibitors: a review of recent patents (2010-2012). Expert Opin Ther Pat. 2013; 23(7):895–909.5. Koskenkorva-Frank TS, Weiss G, Koppenol WH, Burckhardt S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med. 2013; 65:1174–1194.
Article6. Li YH, Yan ZQ, Brauner A, Tullus K. Activation of macrophage nuclear factor-kappa B and induction of inducible nitric oxide synthase by LPS. Respir Res. 2002; 3:23.
Article7. Pekarova M, Lojek A, Martiskova H, Vasicek O, Bino L, Klinke A, Lau D, Kuchta R, Kadlec J, Vrba R, Kubala L. New role for L-arginine in regulation of inducible nitric-oxide-synthase-derived superoxide anion production in RAW264.7 macrophages. ScientificWorldJournal. 2011; 11:2443–2457.8. Ullah MF, Khan MW. Food as medicine: potential therapeutic tendencies of plant derived polyphenolic compounds. Asian Pac J Cancer Prev. 2008; 9(2):187–195.9. Malar DS, Devi KP. Dietary polyphenols for treatment of Alzheimer's disease--future research and development. Curr Pharm Biotechnol. 2014; 15(4):330–342.10. Pérez-Gregorio MR, Regueiro J, Simal-Gándara J, Rodrigues AS, Almeida DP. Increasing the added-value of onions as a source of antioxidant flavonoids: a critical review. Crit Rev Food Sci Nutr. 2014; 54(8):1050–1062.
Article11. Stoclet JC, Schini-Kerth V. Dietary flavonoids and human health. Ann Pharm Fr. 2011; 69(2):78–90.12. El-Gendy MM, Shaaban M, El-Bondkly AM, Shaaban KA. Bioactive benzopyrone derivatives from new recombinant fusant of marine Streptomyces. Appl Biochem Biotechnol. 2008; 150(1):85–96.
Article13. Yu Y, Cai W, Pei CG, Shao Y. Rhamnazin, a novel inhibitor of VEGFR2 signaling with potent antiangiogenic activity and antitumor efficacy. Biochem Biophys Res Commun. 2015; 458(4):913–919.
Article14. Cai H, Xie Z, Liu G, Sun X, Peng G, Lin B, Liao Q. Isolation, identification and activities of natural antioxidants from Callicarpa kwangtungensis Chun. PLoS One. 2014; 9(3):e93000.
Article15. Martini ND, Katerere DR, Eloff JN. Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae). J Ethnopharmacol. 2004; 93(2-3):207–212.
Article16. Huang YC, Guh JH, Cheng ZJ, Chang YL, Hwang TL, Lin CN, Teng CM. Inhibitory effect of DCDC on lipopolysaccharideinduced nitric oxide synthesis in RAW264.7 cells. Life Sci. 2001; 68(21):2435–2447.17. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65(1-2):55–63.
Article18. Paraidathathu T, de Groot H, Kehrer JP. Production of reactive oxygen by mitochondria from normoxic and hypoxic rat heart tissue. Free Radic Biol Med. 1992; 13(4):289–297.
Article19. Kooy NW, Royall JA, Ischiropoulos H, Beckman JS. Peroxynitrite-mediated oxidation of dihydrorhodamine 123. Free Radic Biol Med. 1994; 16(2):149–156.
Article20. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982; 126(1):131–138.
Article21. Ewing JF, Janero DR. Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal Biochem. 1995; 232(2):243–248.
Article22. Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978; 52:302–310.23. Aebi H. Catalase in vitro. Methods Enzymol. 1984; 105:121–126.24. Swaminathan P, Kalva S, Saleena LM. E-pharmacophore and molecular dynamics study of flavonols and dihydroflavonols as inhibitors against dihydroorotate dehydrogenase. Comb Chem High Throughput Screen. 2014; 17(8):663–673.
Article25. Luo C, Wang A, Wang X, Li J, Liu H, Wang M, Wang L, Lai D, Zhou L. A new proline-containing flavonol glycoside from Caragana leucophloea Pojark. Nat Prod Res. 2015; 29(19):1811–1819.26. Grisham MB. Methods to detect hydrogen peroxide in living cells: Possibilities and pitfalls. Comp Biochem Physiol A Mol Integr Physiol. 2013; 165(4):429–438.
Article27. Radak Z, Zhao Z, Goto S, Koltai E. Age-associated neurodegeneration and oxidative damage to lipids, proteins and DNA. Mol Aspects Med. 2011; 32(4-6):305–315.
Article28. Vendramini-Costa DB, Carvalho JE. Molecular link mechanisms between inflammation and cancer. Curr Pharm Des. 2012; 18(26):3831–3852.29. Bai K, Xu W, Zhang J, Kou T, Niu Y, Wan X, Zhang L, Wang C, Wang T. Assessment of free radical scavenging activity of dimethylglycine sodium salt and its role in providing protection against lipopolysaccharide-induced oxidative stress in mice. PLoS One. 2016; 11(5):e0155393.
Article30. Firdous AP, Kuttan G, Kuttan R. Anti-inflammatory potential of carotenoid meso-zeaxanthin and its mode of action. Pharm Biol. 2015; 53(7):961–967.31. Du Z, Liu H, Zhang Z, Li P. Antioxidant and anti-inflammatory activities of Radix Isatidis polysaccharide in murine alveolar macrophages. Int J Biol Macromol. 2013; 58:329–335.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective effect of 6′-Sialyllactose on LPS-induced macrophage inflammation via regulating Nrf2-mediated oxidative stress and inflammatory signaling pathways
- Inhibitory Activity of Cordyceps bassiana Extract on LPS-induced Inflammation in RAW 264.7 Cells by Suppressing NF-κB Activation
- Inhibitory Effect of 3-(4-Hydroxyphenyl)-1-(thiophen-2-yl) prop-2-en-1-one, a Chalcone Derivative on MCP-1 Expression in Macrophages via Inhibition of ROS and Akt Signaling
- The Nuclear Orphan Receptor NR4A1 is Involved in the Apoptotic Pathway Induced by LPS and Simvastatin in RAW 264.7 Macrophages
- YJI-7 Suppresses ROS Production and Expression of Inflammatory Mediators via Modulation of p38MAPK and JNK Signaling in RAW 264.7 Macrophages