Immune Netw.
2014 Apr;14(2):116-122. 10.4110/in.2014.14.2.116.
The Nuclear Orphan Receptor NR4A1 is Involved in the Apoptotic Pathway Induced by LPS and Simvastatin in RAW 264.7 Macrophages
- Affiliations
-
- 1Department of Biochemistry, College of Natural Sciences, Chungnam National University, Daejeon 305-764, Korea. young@cnu.ac.kr
- KMID: 2168022
- DOI: http://doi.org/10.4110/in.2014.14.2.116
Abstract
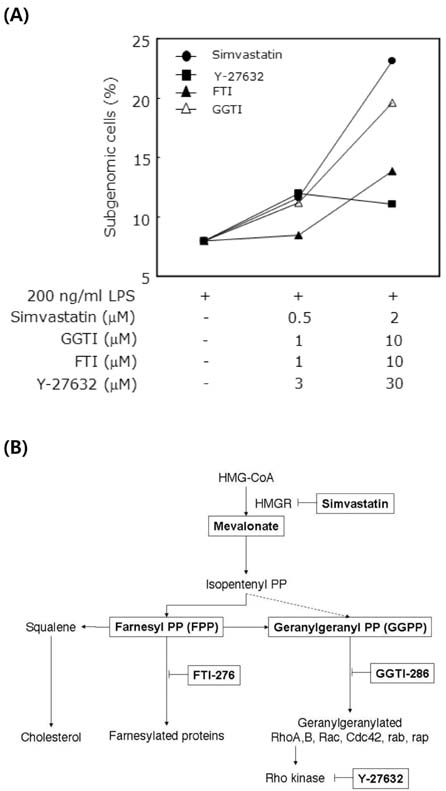
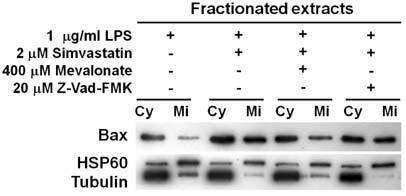
- Macrophage death plays a role in several physiological and inflammatory pathologies such as sepsis and arthritis. In our previous work, we showed that simvastatin triggers cell death in LPS-activated RAW 264.7 mouse macrophage cells through both caspase-dependent and independent apoptotic pathways. Here, we show that the nuclear orphan receptor NR4A1 is involved in a caspase-independent apoptotic process induced by LPS and simvastatin. Simvastatin-induced NR4A1 expression in RAW 264.7 macrophages and ectopic expression of a dominant-negative mutant form of NR4A1 effectively suppressed both DNA fragmentation and the disruption of mitochondrial membrane potential (MMP) during LPS- and simvastatin-induced apoptosis. Furthermore, apoptosis was accompanied by Bcl-2-associated X protein (Bax) translocation to the mitochondria. Our findings suggest that NR4A1 expression and mitochondrial translocation of Bax are related to simvastatin-induced apoptosis in LPS-activated RAW 264.7 macrophages.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
4-(Tert-butyl)-2,6-bis(1-phenylethyl)phenol induces pro-apoptotic activity
Jun Ho Kim, Yunmi Lee, Mi-Yeon Kim, Jae Youl Cho
Korean J Physiol Pharmacol. 2016;20(3):253-259. doi: 10.4196/kjpp.2016.20.3.253.
Reference
-
1. Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990; 343:425–430.
Article2. Cordle A, Koenigsknecht-Talboo J, Wilkinson B, Limpert A, Landreth G. Mechanisms of statin-mediated inhibition of small G-protein function. J Biol Chem. 2005; 280:34202–34209.
Article3. Massy ZA, Keane WF, Kasiske BL. Inhibition of the mevalonate pathway: benefits beyond cholesterol reduction? Lancet. 1996; 347:102–103.
Article4. Sacks FM, Moye LA, Davis BR, Cole TG, Rouleau JL, Nash DT, Pfeffer MA, Braunwald E. Relationship between plasma LDL concentrations during treatment with pravastatin and recurrent coronary events in the Cholesterol and Recurrent Events trial. Circulation. 1998; 97:1446–1452.
Article5. Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999; 100:230–235.
Article6. Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000; 6:1399–1402.
Article7. Weitz-Schmidt G, Welzenbach K, Brinkmann V, Kamata T, Kallen J, Bruns C, Cottens S, Takada Y, Hommel U. Statins selectively inhibit leukocyte function antigen-1 by binding to a novel regulatory integrin site. Nat Med. 2001; 7:687–692.
Article8. Ghittoni R, Patrussi L, Pirozzi K, Pellegrini M, Lazzerini PE, Capecchi PL, Pasini FL, Baldari CT. Simvastatin inhibits T-cell activation by selectively impairing the function of Ras superfamily GTPases. FASEB J. 2005; 19:605–607.
Article9. Yilmaz A, Reiss C, Tantawi O, Weng A, Stumpf C, Raaz D, Ludwig J, Berger T, Steinkasserer A, Daniel WG, Garlichs CD. HMG-CoA reductase inhibitors suppress maturation of human dendritic cells: new implications for atherosclerosis. Atherosclerosis. 2004; 172:85–93.
Article10. Kim YC, Song SB, Lee MH, Kang KI, Lee H, Paik SG, Kim KE, Kim YS. Simvastatin induces caspase-independent apoptosis in LPS-activated RAW264.7 macrophage cells. Biochem Biophys Res Commun. 2006; 339:1007–1014.
Article11. Liang SL, Liu H, Zhou A. Lovastatin-induced apoptosis in macrophages through the Rac1/Cdc42/JNK pathway. J Immunol. 2006; 177:651–656.
Article12. Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995; 83:841–850.
Article13. Martinez-Gonzalez J, Badimon L. The NR4A subfamily of nuclear receptors: new early genes regulated by growth factors in vascular cells. Cardiovasc Res. 2005; 65:609–618.
Article14. Pei L, Castrillo A, Chen M, Hoffmann A, Tontonoz P. Induction of NR4A orphan nuclear receptor expression in macrophages in response to inflammatory stimuli. J Biol Chem. 2005; 280:29256–29262.
Article15. He YW. Orphan nuclear receptors in T lymphocyte development. J Leukoc Biol. 2002; 72:440–446.16. Lee SL, Wesselschmidt RL, Linette GP, Kanagawa O, Russell JH, Milbrandt J. Unimpaired thymic and peripheral T cell death in mice lacking the nuclear receptor NGFI-B (Nur77). Science. 1995; 269:532–535.
Article17. Li H, Kolluri SK, Gu J, Dawson MI, Cao X, Hobbs PD, Lin B, Chen G, Lu J, Lin F, Xie Z, Fontana JA, Reed JC, Zhang X. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science. 2000; 289:1159–1164.
Article18. Winoto A, Littman DR. Nuclear hormone receptors in T lymphocytes. Cell. 2002; 109:Suppl. S57–S66.
Article19. Woronicz JD, Calnan B, Ngo V, Winoto A. Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature. 1994; 367:277–281.
Article20. Bonta PI, van Tiel CM, Vos M, Pols TW, van Thienen JV, Ferreira V, Arkenbout EK, Seppen J, Spek CA, van der Poll T, Pannekoek H, de Vries CJ. Nuclear receptors Nur77, Nurr1, and NOR-1 expressed in atherosclerotic lesion macrophages reduce lipid loading and inflammatory responses. Arterioscler Thromb Vasc Biol. 2006; 26:2288–2294.
Article21. Kim SO, Ono K, Tobias PS, Han J. Orphan nuclear receptor Nur77 is involved in caspase-independent macrophage cell death. J Exp Med. 2003; 197:1441–1452.
Article22. Lee SK, Kim YS. Phosphorylation of eIF2alpha attenuates statin-induced apoptosis by inhibiting the stabilization and translocation of p53 to the mitochondria. Int J Oncol. 2013; 42:810–816.
Article23. Lee SK, Kim YC, Song SB, Kim YS. Stabilization and translocation of p53 to mitochondria is linked to Bax translocation to mitochondria in simvastatin-induced apoptosis. Biochem Biophys Res Commun. 2010; 391:1592–1597.
Article24. Yeo MG, Yoo YG, Choi HS, Pak YK, Lee MO. Negative cross-talk between Nur77 and small heterodimer partner and its role in apoptotic cell death of hepatoma cells. Mol Endocrinol. 2005; 19:950–963.
Article25. Jin BR, Kim SJ, Lee JM, Kang SH, Han HJ, Jang YS, Seo GY, Kim PH. Alum Directly Modulates Murine B Lymphocytes to Produce IgG1 Isotype. Immune Netw. 2013; 13:10–15.
Article26. Lim H, Do SA, Park SM, Kim YS. Tumor Cell Clone Expressing the Membrane-bound Form of IL-12p35 Subunit Stimulates Antitumor Immune Responses Dominated by CD8(+) T Cells. Immune Netw. 2013; 13:63–69.
Article27. Lim HY, Ju HY, Chung HY, Kim YS. Antitumor effects of a tumor cell vaccine expressing a membrane-bound form of the IL-12 p35 subunit. Cancer Biol Ther. 2010; 10:336–343.
Article28. Barish GD, Downes M, Alaynick WA, Yu RT, Ocampo CB, Bookout AL, Mangelsdorf DJ, Evans RM. A Nuclear Receptor Atlas: macrophage activation. Mol Endocrinol. 2005; 19:2466–2477.
Article29. Dijkmans R, Van Damme J, Cornette F, Heremans H, Billiau A. Bacterial lipopolysaccharide potentiates gamma interferon-induced cytotoxicity for normal mouse and rat fibroblasts. Infect Immun. 1990; 58:32–36.
Article30. Yang H, Bushue N, Bu P, Wan YJ. Induction and intracellular localization of Nur77 dictate fenretinide-induced apoptosis of human liver cancer cells. Biochem Pharmacol. 2010; 79:948–954.
Article31. Lin B, Kolluri SK, Lin F, Liu W, Han YH, Cao X, Dawson MI, Reed JC, Zhang XK. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell. 2004; 116:527–540.
Article32. Masuyama N, Oishi K, Mori Y, Ueno T, Takahama Y, Gotoh Y. Akt inhibits the orphan nuclear receptor Nur77 and T-cell apoptosis. J Biol Chem. 2001; 276:32799–32805.
Article33. Rajpal A, Cho YA, Yelent B, Koza-Taylor PH, Li D, Chen E, Whang M, Kang C, Turi TG, Winoto A. Transcriptional activation of known and novel apoptotic pathways by Nur77 orphan steroid receptor. EMBO J. 2003; 22:6526–6536.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Regulatory Mechanism of Src Familly Kinase in Lipopolysaccharide (LPS) induced HF-kappaB Activation Pathway
- Suppression of Heme Oxygenase-1 by Prostaglandin E2-Protein Kinase A-A-Kinase Anchoring Protein Signaling Is Central for Augmented Cyclooxygenase-2 Expression in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages
- Function of bcl-X proteins in Nitric Oxide-induced Apoptosis in RAW 264.7 Macrophages
- Induction Mechanism of PD-L1 (Programmed Cell Death-ligand 1) in Sepsis
- The Roles of SEK1 in Nitric Oxide (NO) Induced Apoptsis of RAW264.7 cells