Korean J Physiol Pharmacol.
2024 Nov;28(6):503-513. 10.4196/kjpp.2024.28.6.503.
Protective effect of 6′-Sialyllactose on LPS-induced macrophage inflammation via regulating Nrf2-mediated oxidative stress and inflammatory signaling pathways
- Affiliations
-
- 1College of Pharmacy and Institute of Drug Research and Development, Chungnam National University, Daejeon 34134, Korea
- 2GeneChem Inc., Daejeon 34025, Korea
- 3Department of Cancer AI & Digital Health, National Cancer Center, Goyang 10408, Korea
- KMID: 2560730
- DOI: http://doi.org/10.4196/kjpp.2024.28.6.503
Abstract
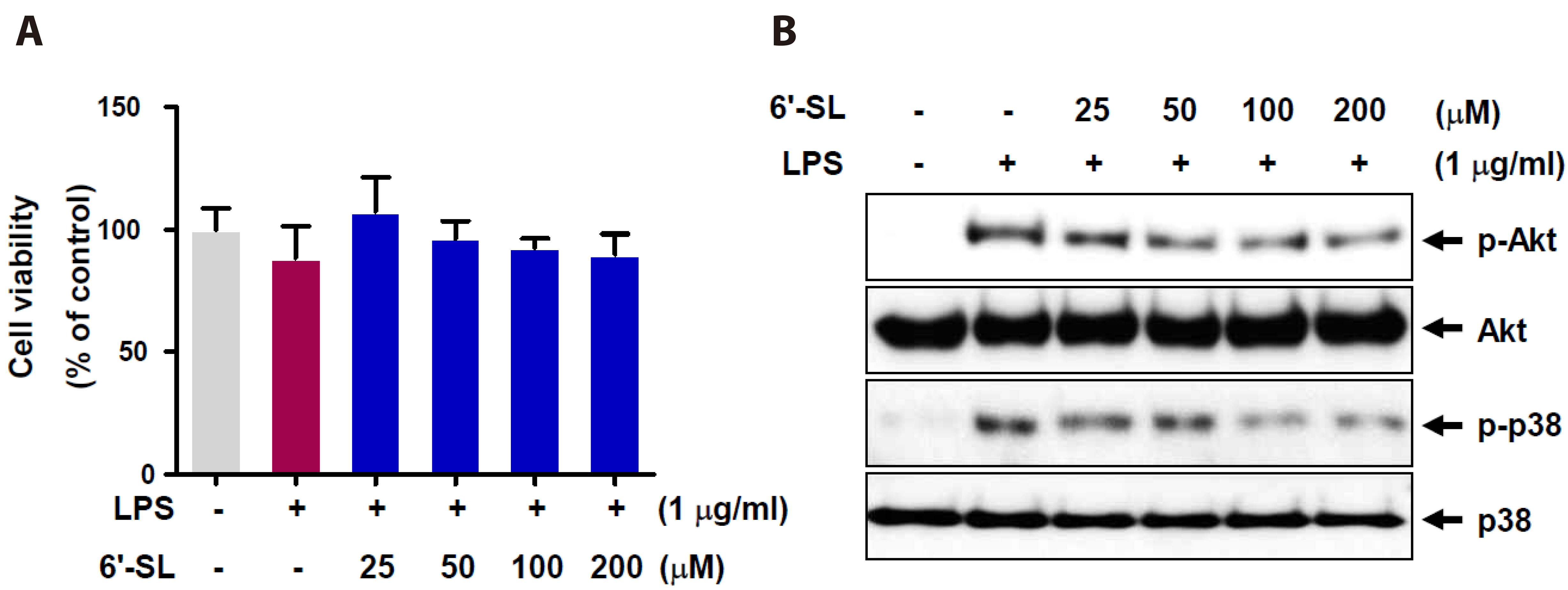
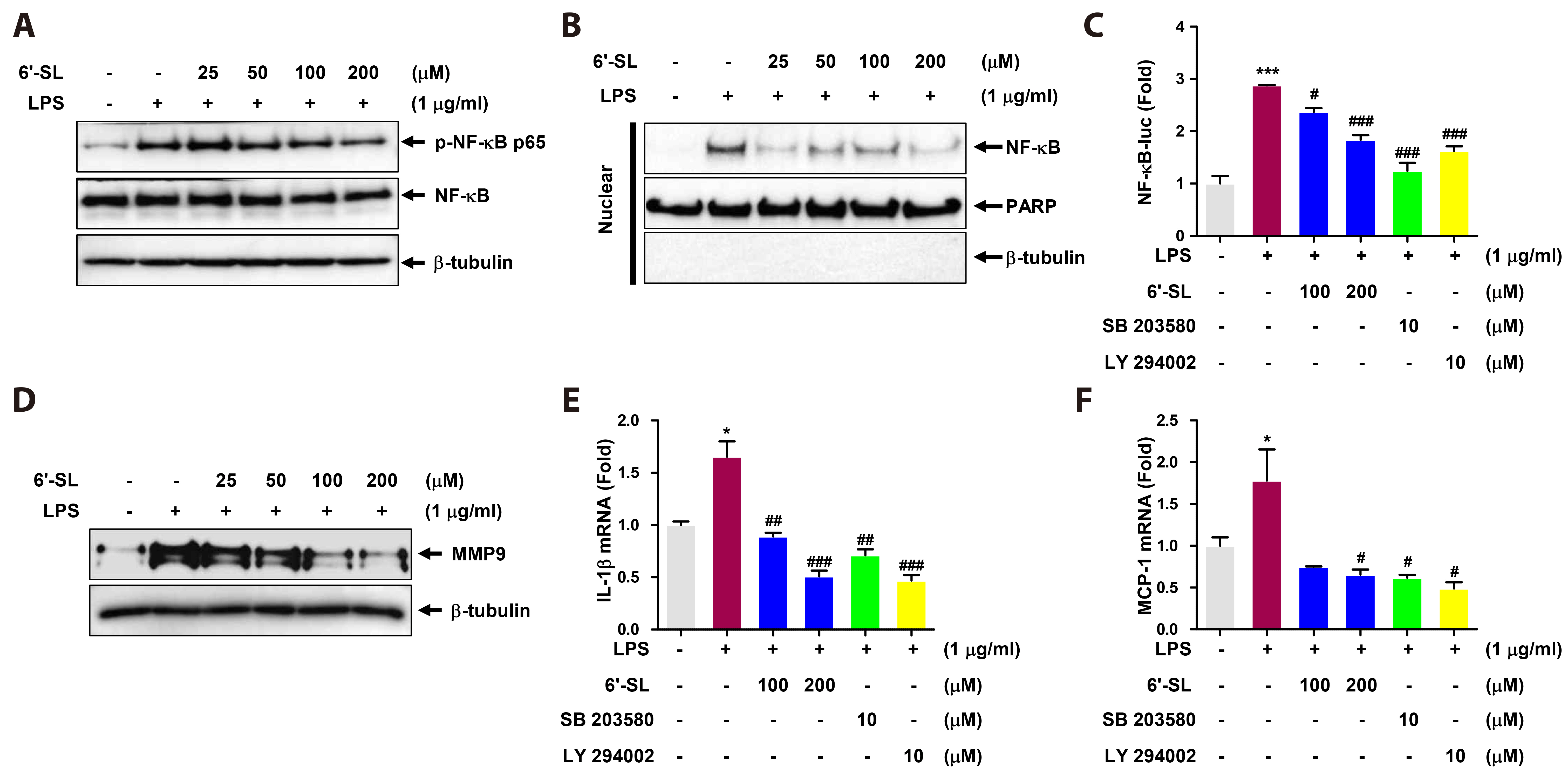
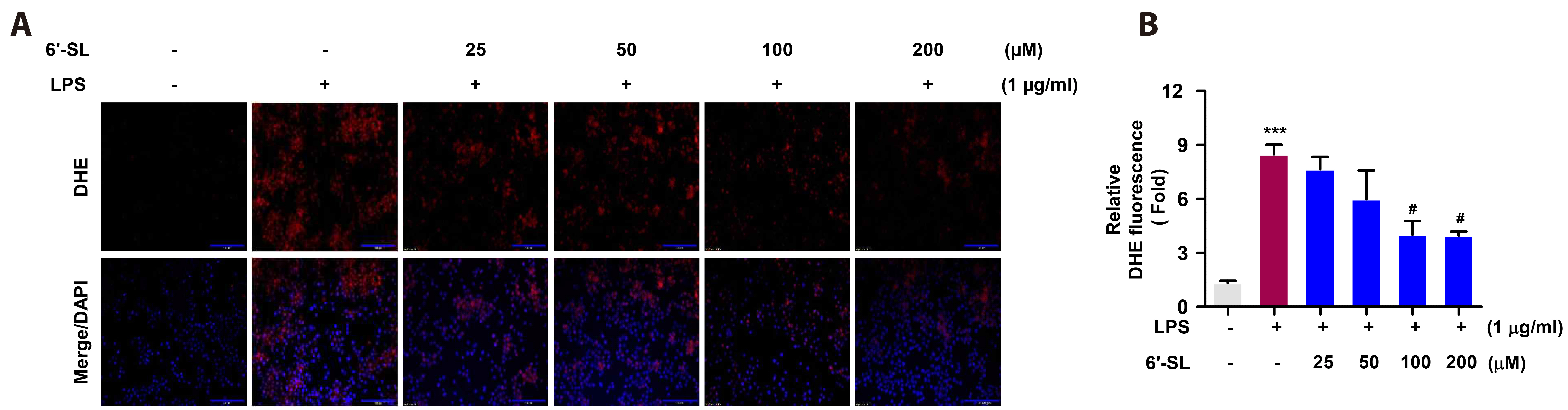
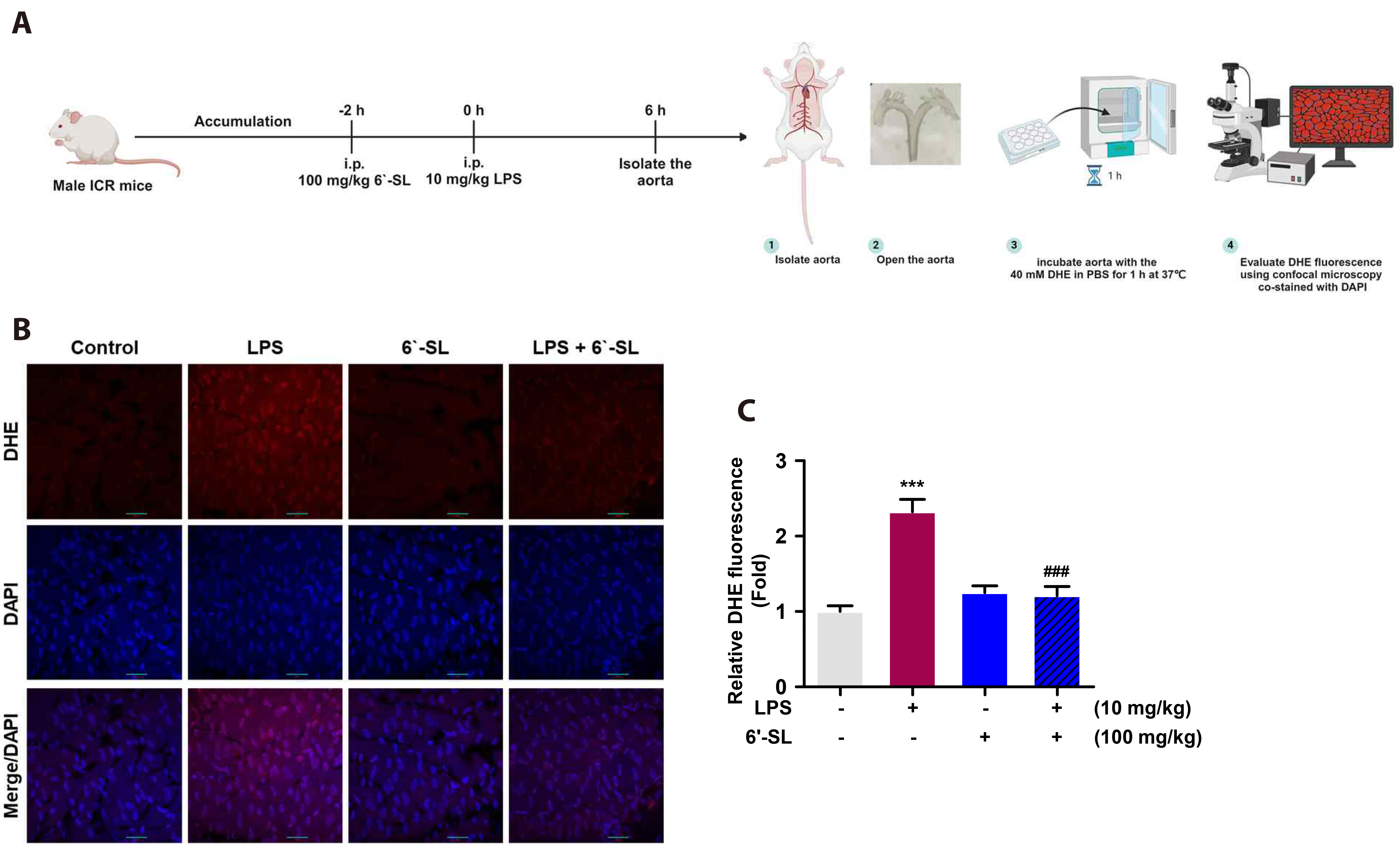
- Macrophages play a central role in cardiovascular diseases, like atherosclerosis, by accumulating in vessel walls and inducing sustained local inflammation marked by the release of chemokines, cytokines, and matrix-degrading enzymes. Recent studies indicate that 6'-sialyllactose (6'-SL) may mitigate inflammation by modulating the immune system. Here, we examined the impact of 6'-SL on lipopolysaccharide (LPS)-induced acute inflammation using RAW 264.7 cells and a mouse model. In vivo, ICR mice received pretreatment with 100 mg/kg 6'-SL for 2 h, followed by intraperitoneal LPS injection (10 mg/kg) for 6 h. In vitro, RAW 264.7 cells were preincubated with 6'-SL before LPS stimulation. Mechanistic insights were gained though Western blotting, qRT-PCR, and immunofluorescence analysis, while reactive oxygen species (ROS) production was assessed via DHE assay. 6'-SL effectively attenuated LPS-induced p38 MAPK and Akt phosphorylation, as well as p65 nuclear translocation. Additionally, 6'-SL inhibited LPS-induced expression of tissue damage marker MMP9, IL-1β, and MCP-1 by modulating NF-κB activation. It also reduced ROS levels, mediated by p38 MAPK and Akt pathways. Moreover, 6'-SL restored LPS-suppressed Nrf2 and HO-1 akin to specific inhibitors SB203580 and LY294002. Consistent with in vitro results, 6'-SL decreased oxidative stress, MMP9, and MCP-1 expression in mouse endothelium following LPS-induced macrophage activation. In summary, our findings suggest that 6'-SL holds promise in mitigating atherosclerosis by dampening LPS-induced acute macrophage inflammation.
Figure
Reference
-
1. Eshghjoo S, Kim DM, Jayaraman A, Sun Y, Alaniz RC. 2022; Macrophage polarization in atherosclerosis. Genes (Basel). 13:756. DOI: 10.3390/genes13050756. PMID: 35627141. PMCID: PMC9142092.2. Hansson GK, Hermansson A. 2011; The immune system in atherosclerosis. Nat Immunol. 12:204–212. DOI: 10.1038/ni.2001. PMID: 21321594.3. Jin Y, Heo KS. 2023; Experimental model and novel therapeutic targets for non-alcoholic fatty liver disease development. Korean J Physiol Pharmacol. 27:299–310. DOI: 10.4196/kjpp.2023.27.4.299. PMID: 37386828. PMCID: PMC10316197.4. Jin Y, Jeon H, Le Lam Nguyen T, Kim L, Heo KS. 2023; Human milk oligosaccharides 3'-sialyllactose and 6'-sialyllactose attenuate LPS-induced lung injury by inhibiting STAT1 and NF-κB signaling pathways. Arch Pharm Res. 46:897–906. DOI: 10.1007/s12272-023-01470-1. PMID: 37940817.5. Jin Y, Tangchang W, Kwon OS, Lee JY, Heo KS, Son HY. 2023; Ginsenoside Rh1 ameliorates the asthma and allergic inflammation via inhibiting Akt, MAPK, and NF-κB signaling pathways in vitro and in vivo. Life Sci. 321:121607. DOI: 10.1016/j.lfs.2023.121607. PMID: 36958436.6. Zhang JM, An J. 2007; Cytokines, inflammation, and pain. Int Anesthesiol Clin. 45:27–37. DOI: 10.1097/AIA.0b013e318034194e. PMID: 17426506. PMCID: PMC2785020.7. Huynh DT, Heo KS. 2022; Therapeutic effects of ginsenosides on vascular smooth muscle cell phenotypic switching in vascular diseases. Cardiometab Syndr J. 2:96–107. DOI: 10.51789/cmsj.2022.2.e16.8. Nguyen DV, Jin Y, Nguyen TLL, Kim L, Heo KS. 2024; 3'-Sialyllactose protects against LPS-induced endothelial dysfunction by inhibiting superoxide-mediated ERK1/2/STAT1 activation and HMGB1/RAGE axis. Life Sci. 338:122410. DOI: 10.1016/j.lfs.2023.122410. PMID: 38191050.9. Nguyen TLL, Jin Y, Kim L, Heo KS. 2022; Inhibitory effects of 6'-sialyllactose on angiotensin II-induced proliferation, migration, and osteogenic switching in vascular smooth muscle cells. Arch Pharm Res. 45:658–670. DOI: 10.1007/s12272-022-01404-3. PMID: 36070173.10. Chen Y, Waqar AB, Nishijima K, Ning B, Kitajima S, Matsuhisa F, Chen L, Liu E, Koike T, Yu Y, Zhang J, Chen YE, Sun H, Liang J, Fan J. 2020; Macrophage-derived MMP-9 enhances the progression of atherosclerotic lesions and vascular calcification in transgenic rabbits. J Cell Mol Med. 24:4261–4274. DOI: 10.1111/jcmm.15087. PMID: 32126159. PMCID: PMC7171347.11. Huynh DTN, Baek N, Sim S, Myung CS, Heo KS. 2020; Minor ginsenoside Rg2 and Rh1 attenuates LPS-induced acute liver and kidney damages via downregulating activation of TLR4-STAT1 and inflammatory cytokine production in macrophages. Int J Mol Sci. 21:6656. DOI: 10.3390/ijms21186656. PMID: 32932915. PMCID: PMC7555743.12. Jin Y, Nguyen TLL, Myung CS, Heo KS. 2022; Ginsenoside Rh1 protects human endothelial cells against lipopolysaccharide-induced inflammatory injury through inhibiting TLR2/4-mediated STAT3, NF-κB, and ER stress signaling pathways. Life Sci. 309:120973. DOI: 10.1016/j.lfs.2022.120973. PMID: 36150463.13. Jeon H, Jin Y, Myung CS, Heo KS. 2021; Ginsenoside-Rg2 exerts anti-cancer effects through ROS-mediated AMPK activation associated mitochondrial damage and oxidation in MCF-7 cells. Arch Pharm Res. 44:702–712. DOI: 10.1007/s12272-021-01345-3. PMID: 34302638.14. Nguyen TLL, Nguyen DV, Heo KS. 2024; Potential biological functions and future perspectives of sialylated milk oligosaccharides. Arch Pharm Res. 47:325–340. DOI: 10.1007/s12272-024-01492-3. PMID: 38561494.15. Van Nguyen D, Nguyen TLL, Jin Y, Kim L, Myung CS, Heo KS. 2022; 6'-Sialylactose abolished lipopolysaccharide-induced inflammation and hyper-permeability in endothelial cells. Arch Pharm Res. 45:836–848. DOI: 10.1007/s12272-022-01415-0. PMID: 36401777.16. Chang JW, Kim S, Lee EY, Leem CH, Kim SH, Park CS. 2022; Cell-cell contacts via N-cadherin induce a regulatory renin secretory phenotype in As4.1 cells. Korean J Physiol Pharmacol. 26:479–499. DOI: 10.4196/kjpp.2022.26.6.479. PMID: 36302623. PMCID: PMC9614399.17. Huynh DTN, Heo KS. 2022; Rh1 abolishes MCF-7 cell growth via down-regulation of ROS-induced PKCδ/p38/ERK1/2 signaling pathway. Drug Targets and Therapeutics. 1:19–26. DOI: 10.58502/DTT.22.009.18. Jang EJ, Kim H, Baek SE, Jeon EY, Kim JW, Kim JY, Kim CD. 2022; HMGB1 increases RAGE expression in vascular smooth muscle cells via ERK and p-38 MAPK-dependent pathways. Korean J Physiol Pharmacol. 26:389–396. DOI: 10.4196/kjpp.2022.26.5.389. PMID: 36039739. PMCID: PMC9437367.19. Meng RY, Li CS, Hu D, Kwon SG, Jin H, Chai OH, Lee JS, Kim SM. 2023; Inhibition of the interaction between Hippo/YAP and Akt signaling with ursolic acid and 3'3-diindolylmethane suppresses esophageal cancer tumorigenesis. Korean J Physiol Pharmacol. 27:493–511. DOI: 10.4196/kjpp.2023.27.5.493. PMID: 37641811. PMCID: PMC10466072.20. Nguyen TLL, Huynh DTN, Jin Y, Jeon H, Heo KS. 2021; Protective effects of ginsenoside-Rg2 and -Rh1 on liver function through inhibiting TAK1 and STAT3-mediated inflammatory activity and Nrf2/ARE-mediated antioxidant signaling pathway. Arch Pharm Res. 44:241–252. DOI: 10.1007/s12272-020-01304-4. PMID: 33537886.21. Gough PJ, Gomez IG, Wille PT, Raines EW. 2006; Macrophage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 116:59–69. DOI: 10.1172/JCI25074. PMID: 16374516. PMCID: PMC1319218.22. Li T, Li X, Feng Y, Dong G, Wang Y, Yang J. 2020; The role of matrix metalloproteinase-9 in atherosclerotic plaque instability. Mediators Inflamm. 2020:3872367. DOI: 10.1155/2020/3872367. PMID: 33082709. PMCID: PMC7557896.23. Bobryshev YV, Ivanova EA, Chistiakov DA, Nikiforov NG, Orekhov AN. 2016; Macrophages and their role in atherosclerosis: pathophysiology and transcriptome analysis. Biomed Res Int. 2016:9582430. DOI: 10.1155/2016/9582430. PMID: 27493969. PMCID: PMC4967433.24. Wang Y, Liu H, Zhao J. 2020; Macrophage polarization induced by probiotic bacteria: a concise review. Probiotics Antimicrob Proteins. 12:798–808. DOI: 10.1007/s12602-019-09612-y. PMID: 31741313.25. Li J, Qin Y, Chen Y, Zhao P, Liu X, Dong H, Zheng W, Feng S, Mao X, Li C. 2020; Mechanisms of the lipopolysaccharide-induced inflammatory response in alveolar epithelial cell/macrophage co-culture. Exp Ther Med. 20:76. DOI: 10.3892/etm.2020.9204. PMID: 32968433. PMCID: PMC7500047.26. Tsai CF, Chen GW, Chen YC, Shen CK, Lu DY, Yang LY, Chen JH, Yeh WL. 2021; Regulatory effects of quercetin on M1/M2 macrophage polarization and oxidative/antioxidative balance. Nutrients. 14:67. DOI: 10.3390/nu14010067. PMID: 35010945. PMCID: PMC8746507.27. Tan HY, Wang N, Li S, Hong M, Wang X, Feng Y. 2016; The reactive oxygen species in macrophage polarization: reflecting its dual role in progression and treatment of human diseases. Oxid Med Cell Longev. 2016:2795090. DOI: 10.1155/2016/2795090. PMID: 27143992. PMCID: PMC4837277.28. Saha S, Buttari B, Panieri E, Profumo E, Saso L. 2020; An overview of Nrf2 signaling pathway and its role in inflammation. Molecules. 25:5474. DOI: 10.3390/molecules25225474. PMID: 33238435. PMCID: PMC7700122.29. Perdiguero E, Sousa-Victor P, Ruiz-Bonilla V, Jardí M, Caelles C, Serrano AL, Muñoz-Cánoves P. 2011; p38/MKP-1-regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. J Cell Biol. 195:307–322. DOI: 10.1083/jcb.201104053. PMID: 21987635. PMCID: PMC3198158.30. Ci X, Zhou J, Lv H, Yu Q, Peng L, Hua S. 2017; Betulin exhibits anti-inflammatory activity in LPS-stimulated macrophages and endotoxin-shocked mice through an AMPK/AKT/Nrf2-dependent mechanism. Cell Death Dis. 8:e2798. DOI: 10.1038/cddis.2017.39. PMID: 28518138. PMCID: PMC5520743.31. Yang H, Lv H, Li H, Ci X, Peng L. 2019; Oridonin protects LPS-induced acute lung injury by modulating Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-κB pathways. Cell Commun Signal. 17:62. DOI: 10.1186/s12964-019-0366-y. PMID: 31186013. PMCID: PMC6558832.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Oligomeric proanthocyanidin ameliorates sepsis-associated renal tubular injury: involvement of oxidative stress, inflammation, PI3K/AKT and NFκκB signaling pathways
- Rhamnazin inhibits LPS-induced inflammation and ROS/RNS in raw macrophages
- Carbon monoxide releasing molecule-2 protects mice against acute kidney injury through inhibition of ER stress
- Suppression of Lipopolysaccharide-Induced Inflammatory and Oxidative Response by 5-Aminolevulinic Acid in RAW 264.7 Macrophages and Zebrafish Larvae
- Protopanaxatriol Ginsenoside Rh1 Upregulates Phase II Antioxidant Enzyme Gene Expression in Rat Primary Astrocytes: Involvement of MAP Kinases and Nrf2/ARE Signaling