Establishment for Reference Range of Serum HER-2/neu in Korean Healthy Women
- Affiliations
-
- 1Department of Surgery, Soonchunhyang University, College of Medicine, Seoul, Korea. mhlee@hosp.sch.ac.kr
- 2Department of Emergency Medicine, Soonchunhyang University, College of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, Soonchunhyang University, College of Medicine, Seoul, Korea.
- KMID: 2286601
- DOI: http://doi.org/10.4048/jbc.2006.9.4.301
Abstract
-
PURPOSE: HER-2/neu oncogene is known to play a part in the process of carcinogenesis, while the biological characteristics of HER-2/neu oncoprotein include regulating cell growth and increasing the reproductionability of a tumor. The extracelluar domain (ECD), whose molecular weight is between 95 and 105 kD among the HER-2/neu oncoprotein structures, is proteolyzed and separated from the cell surface by metalloproteases and goes into the blood stream where it starts circulation. Since monoclonal antibody was developed for the serum HER-2/neu ECD, it's now possible to measure HER-2/neu ECD in the serum with the immunoassay method. The measurement of serum HER-2/neu ECD is used for prognosis of metastatic breast cancer and for testing the treating effect of trastuzumab (Herceptin(R)), a target agent for the patients positive to the HER-2/neu receptor. In Korea there is no report on the accurate reference range of serum HER-2/neu for healthy women. The purposes of this study were to measure the serum HER-2/neu ECD in healthy Korean women, analyze the reference range.
METHODS
The subjects of the study include 200 healthy Korean women with 50 from each in their twenties, thirties, forties, and fifties. As for methodology, the HER-2/neu in the serum separated from their blood was measured. The serum HER-2/neu level was measured quantitatively with the recently developed ADVIA Centaur(R) automated immunoassay analyzer and ADVIA Centaur(R) HER-2/neu assay reagent. With the measurement, you can use the sandwich immunoassay and direct chemiluminescence technique for two monoclonal antibodies for the epitopes located in the serum HER-2/neu ECD. The reference ranges were calculated based on the mean +/- 2 SD.
RESULTS
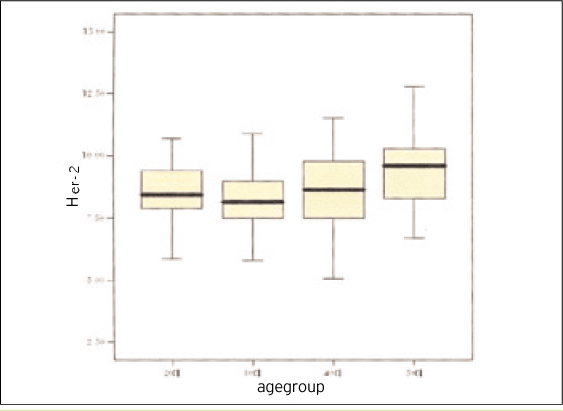
One of the 200 healthy subjects was excluded from analysis for having the highest value of serum HER-2/neu (23.1 ng/ml), and the data of total 199 were used for analysis. The analysis results indicated that the minimum value was 3.5 ng/mL, the maximum value 14.5 ng/mL, the mean 8.6 ng/mL, average 8.77 ng/mL, and SD 1.61 ng/mL. The reference range of the 199 subjects's serum HER-2/neu measurements was calculated by the mean +/- 2 SD. Since the mean +/- SD of their HER-2/neu measurements was 8.8 +/- 1.6 ng/mL, the reference range was 5.6~12.0 ng/mL. The reference ranges for the age groups were 6.1~10.9 ng/mL, 5.3~11.4 ng/mL, 5.0~12.6 ng/mL and 6.3~12.6 ng/mL for the twenties, thirties, forties and fifties, respectively. The reference ranges for the age groups were analyzed statistically and there was statistical difference (p= 0.002) between fifties and twenties or thirties. The upper limit level of the reference range of serum HER-2/neu in healthy Korean women was 12.0 ng/mL.
CONCLUSION
The results suggest that the reference range of serum HER-2/neu in healthy Korean women is 6.1~10.9 ng/mL, 5.3~11.4 ng/mL, 5.0~12.6 ng/mL and 6.3~12.6 ng/mL for the twenties, thirties, forties, and fifties, respectively. There is no significant difference between the twenties, thirties, forties, each other. According to analyzed statistically, there is difference between fifties and twenties or thirties (p= 0.002), but there is no statistically significant difference between forties and fifties.
Keyword
MeSH Terms
Figure
Cited by 3 articles
-
Correlation between the Her-2/neu Status as Determined by Immunohistochemical Analysis and the Serum Her-2/neu Concentration as Determined by the Use of ADVIA Cencaur® Automated Immunoassay in Breast Cancer Patients
Jung-Sun Lee, Won Ki Min, Eun Hwa Park, Woo-Sung Lim, Sung-Lim Choi, Byung-Ho Son, Sung-Bae Kim, Jin-Hee Ahn, Sei-Hyun Ahn
J Breast Cancer. 2008;11(3):116-124. doi: 10.4048/jbc.2008.11.3.116.The Correlation of Serum HER-2/neu and CA15-3 in Patients with Metastatic Breast Cancer
Nae-Kyeong Park, Hee-Doo Woo, Doo-Min Sohn, Sung-Yong Kim, Cheol-Wan Lim, Tae-Youn Choi, Jae-Jun Kim, Min-Hyuk Lee
J Breast Cancer. 2008;11(1):18-24. doi: 10.4048/jbc.2008.11.1.18.Impact of Serum HER2 Levels on Survival and Its Correlation with Clinicopathological Parameters in Women with Breast Cancer
Dong Won Ryu, Chung Han Lee
J Breast Cancer. 2012;15(1):71-78. doi: 10.4048/jbc.2012.15.1.71.
Reference
-
1. Bargmann CI, Hung MC, Weinberg RA. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986. 319:226–230.
Article2. Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, McGrath J, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985. 230:1132–1139.
Article3. Guerin M, Gabillot M, Mathieu MC, Travagli JP, Spielmann M, Andrieu N, et al. Structure and expression of c-erbB-2 and EGF receptor genes in inflammatory and non-inflammatory breast cancer: prognostic significance. Int J Cancer. 1989. 43:201–208.
Article4. Gullick WJ, Bottomley AC, Lofts FJ, Doak DG, Mulvey D, Newman R, et al. Three dimensional structure of the transmembrane region of the proto-oncogenic and oncogenic forms of the neu protein. EMBO J. 1992. 11:43–48.
Article5. Neve RM, Lane HA, Hynes NE. The role of over expressed HER2 in transformation. Ann Oncol. 2001. 12:Suppl 1. S9–S13.6. Hynes NE. Amplification and overexpression of the erbB-2 gene in human tumors: its involvement in tumor development, significance as a prognostic factor, and potential as a target for cancer therapy. Semin Cancer Biol. 1993. 4:19–26.7. Nunes RA, Harris LN. The HER2 extracellular domain as a prognostic and predictive factor in breast cancer. Clin Breast Cancer. 2002. 3:125–137.
Article8. Chi DS, Liao JB, Leon LF, Venkatraman ES, Hensley ML, Bhaskaran D, et al. dentification of prognostic factors in advanced epithelial ovarian carcinoma. Gynecol Oncol. 2001. 82:532–537.
Article9. Pinto-de-Sousa J, David L, Almeida R, Leitao D, Preto JR, Seixas M, et al. c-erb B-2 expression is associated with tumor location and venous invasion and influences survival of patients with gastric carcinoma. Int J Surg Pathol. 2002. 10:247–256.
Article10. Potti A, Willardson J, Forseen C, Kishor Ganti A, Koch M, Hebert B, et al. Predictive role of HER-2/neu overexpression and clinical features at initial presentation in patients with extensive stage small cell lung carcinoma. Lung Cancer. 2002. 36:257–261.
Article11. Ariga R, Zarif A, Korasick J, Reddy V, Siziopikou K, Gattuso P. Correlation of HER-2/neu gene amplification with other prognostic and predictive factors in female breast carcinoma. Breast J. 2005. 11:278–280.
Article12. Stal O, Sullivan S, Wingren S, Skoog L, Rutqvist LE, Carstensen JM, et al. c-erbB-2 expression and benefit from adjuvant chemotherapy and radiotherapy of breast cancer. Eur J Cancer. 1995. 31:2185–2190.
Article13. Benz CC, Scott GK, Sarup JC, Johnson RM, Tripathy D, Coronado E, et al. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res Treat. 1993. 24:85–95.
Article14. Sawaki M, Ito Y, Tada K, Mizunuma N, Takahashi S, Horikoshi N, et al. Efficacy and safety of trastuzumab as a single agent in heavily pretreated patients with HER-2/neu-overexpressing metastatic breast cancer. Tumori. 2004. 90:40–43.
Article15. Pauletti G, Dandekar S, Rong H, Ramos L, Peng H, Seshadri R, et al. Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: a direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol. 2000. 18:3651–3664.
Article16. Codony-Servat J, Albanell J, Lopez-Talavera JC, Arribas J, Baselga J. Cleavage of the HER2 ectodomain is a pervanadate-activable process that is inhibited by the tissue inhibitor of metalloproteases-1 in breast cancer cells. Cancer Res. 1999. 59:1196–1201.17. Cook GB, Neaman IE, Goldblatt JL, Cambetas DR, Hussain M, Luftner D, et al. Clinical utility of serum HER-2/neu testing on the Bayer Immuno 1 automated system in breast cancer. Anticancer Res. 2001. 21:1465–1470.18. Meenakshi A, Kumar RS, Kumar NS. ELISA for quantitation of serum C-erbB-2 oncoprotein in breast cancer patients. J Immunoassay Immunochem. 2002. 23:293–305.
Article19. Hayes DF, Yamauchi H, Broadwater G, Cirrincione CT, Rodrigue SP, Berry DA, et al. Cancer and Leukemia Group B. Circulating HER-2/erbB-2/c-neu (HER-2) extracellular domain as a prognostic factor in patients with metastatic breast cancer: Cancer and Leukemia Group B Study 8662. Clin Cancer Res. 2001. 7:2703–2711.20. Lipton A, Ali SM, Leitzel K, Demers L, Chinchilli V, Engle L, et al. Elevated serum HER-2/neu level predicts decreased response to hormone therapy in metastatic breast cancer. J Clin Oncol. 2002. 20:1467–1472.
Article21. Muller V, Witzel I, Luck HJ, Kohler G, von Minckwitz G, Mobus V, et al. Prognostic and predictive impact of the HER-2/ neu extracellular domain (ECD) in the serum of patients treated with chemotherapy for metastatic breast cancer. Breast Cancer Res Treat. 2004. 86:9–18.
Article22. Kostler WJ, Schwab B, Singer CF, neumann R, Rucklinger E, Brodowicz T, et al. Monitoring of serum HER-2/neu predicts response and progression-free survival to trastuzumab-based treatment in patients with metastatic breast cancer. Clin Cancer Res. 2004. 10:1618–1624.
Article23. Molina R, Jo J, Filella X, Zanon G, Farrus B, Munoz M, et al. C-erbB-2, CEA and CA 15.3 serum levels in the early diagnosis of recurrence of breast cancer patients. Anticancer Res. 1999. 19:2551–2555.24. Ali SM, Leitzel K, Chinchilli VM, Engle L, Demers L, Harvey HA, et al. Relationship of serum HER-2/neu and serum CA 15-3 in patients with metastatic breast cancer. Clin Chem. 2002. 48:1314–1320.
Article25. Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Ferenbacher L, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER-2 overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999. 17:2639–2648.26. Kim SY, Kim TY, Kim JJ, Kim CH, Song OP, Lee MH, et al. Clinical correlation of HER-2/neu overexpression in patients with breast cancer. J Korean Breast Cancer Society. 2004. 7:244–250.
Article27. Burstein HJ, Kuter I, Campos SM, Gelman RS, Tribou L, Parker LM, et al. Clinical activity of trastuzumab and vinorelbine in women with HER-2/neu-overexpressing metastastic breast cancer. J Clin Oncol. 2001. 19:2722–2730.
Article28. Saceda M, Grunt TW, Colomer R, Lippman ME, Lupu R, Martin MB. Regulation of estrogen receptor concentration and activity by an erbB/HER ligand in breast carcinoma cell lines. Endoclinology. 1996. 137:4322–4330.
Article29. Pietras RJ, Arboleda J, Reese DM, Wongvipat N, Pegram MD, Ramos L, et al. HER-2 tyrosine kinase pathway targets estrogen receptor and promotes hormone-independent growth in humane breast cancer cell. Oncogene. 1995. 10:2435–2446.30. Luftner D, Cheli C, Mickelson K, Sampson E, Possinger K. ADVIA Centaur HER-2/neu shows value in monitoring patients with metastatic breast cancer. Int J Biol Markers. 2004. 19:175–182.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pilot Study of the Clinical Significance of Serum and Urinary HER-2/neu Protein in Bladder Cancer Patients
- The Correlation of Serum HER-2/neu and CA15-3 in Patients with Metastatic Breast Cancer
- Correlation between the Her-2/neu Status as Determined by Immunohistochemical Analysis and the Serum Her-2/neu Concentration as Determined by the Use of ADVIA Cencaur(R) Automated Immunoassay in Breast Cancer Patients
- The Predictive Value of Serum HER2/neu for Response to Anthracycline-Based and Trastuzumab-Based Neoadjuvant Chemotherapy
- Evaluation for the Clinical Usefulness of Serum HER-2/neu Oncoprotein in Patients with Breast Cancer