J Breast Cancer.
2008 Mar;11(1):18-24. 10.4048/jbc.2008.11.1.18.
The Correlation of Serum HER-2/neu and CA15-3 in Patients with Metastatic Breast Cancer
- Affiliations
-
- 1Department of Surgery and 1Laboratory Medicine, Soonchunhyang University, College of Medicine, Seoul, Korea. mhlee@hosp.sch.ac.kr
- KMID: 2175043
- DOI: http://doi.org/10.4048/jbc.2008.11.1.18
Abstract
-
PURPOSE: The extracellular domain (ECD) of the HER-2/neu oncoprotein, whose molecular weight is the range from the 95 kD to 105 kD, is shed into the blood from the cell surface via, proteolysis by a metalloprotease. A monoclonal antibody immunoassay has been developed for measuring the circulating concentrations of serum HER-2/neu ECD (following serum HER-2/neu). Serum HER-2/neu has been reported to be correlated with an increased tumor volume in those patients suffering with breast cancer. We measured the serum CA15-3 level, which is a surrogate marker of the tumor burden, we analyzed the correlation of the serum CA15-3 with the serum HER-2/neu and we analyzed the association of both markers with the clinical outcomes.
METHODS
The sera for the analysis of both HER-2/neu and CA15-3 were obtained from 99 healthy Korean women, 66 primary breast cancer patients and 43 metastatic breast caner patients. The serum HER-2/neu level was measured quantitatively with using an ADVIA Centaur(R) automated immunoassay analyzer (Bayer Health Care LLC, Diagnostics Division, Tarrytown, New York, USA) and the CA 15-3 level was measured via radioimmunoassay.
RESULTS
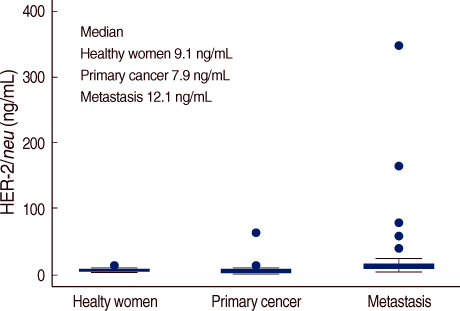
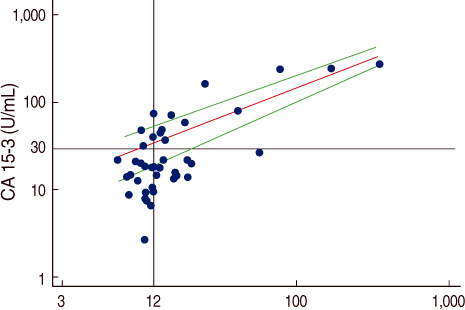
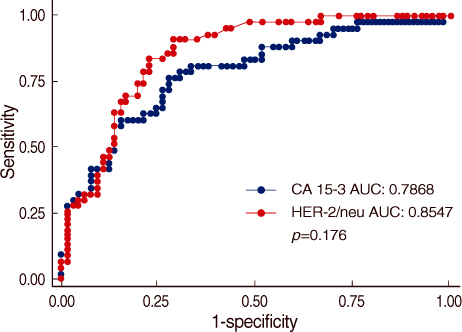
The serum HER-2/neu level was increased 23 metastatic cancer patients (53%). On the analysis of the correlation of serum HER-2/neu and CA15-3, the correlation coefficient (r) was 0.8072. Thus a positive serum HER-2/neu test in breast cancer patients was highly associated with the CA15-3 level for assessing whether metastasis was present or not. For the relationship between primary breast cancer and metastatic breast cancer, the former was classified as the control group and the latter as the patient group. The results of the Receiver Operation Characteristic (ROC) curve for serum HER-2/neu and CA15-3 showed no statistically significant differences (p=0.176) but the diagnostic efficacy of the serum HER-2/neu test was measured more exactly than that of CA15-3 and CA15-3 a tool for measuring a tumor marker for the diagnosis of whether metastasis was present or not.
CONCLUSION
Serum HER-2/neu is a significant independent predictive prognostic factor for metastatic breast cancer patients. In view of the results we have achieved so far the serum HER-2/neu level in metastatic breast cancer patients may play an important roll as an independent tumor marker.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Impact of Serum HER2 Levels on Survival and Its Correlation with Clinicopathological Parameters in Women with Breast Cancer
Dong Won Ryu, Chung Han Lee
J Breast Cancer. 2012;15(1):71-78. doi: 10.4048/jbc.2012.15.1.71.
Reference
-
1. Korean Breast Cancer Society. Nationwide Korean breast cancer data 2002. J Breast Cancer. 2004. 7:72–83.2. Michael JD. Serum tumor markers in breast cancer: are they of clinical value? Clin Chem. 2006. 52:345–351.
Article3. Bates SE. Clinical applications of serum tumour markers. Ann Intern Med. 1991. 115:623–638.4. Beastall GH, Cook B, Rustin GJ, Jennings J. A review of the role of established tumour markers. Ann Clin Biochem. 1991. 28:5–18.
Article5. Gang Y, Adachi I, Okhura H, Yamomoto H, Mizuguchi Y, Abe K. CA15-3 is present as a novel tumour marker in the sera of patients with breast cancer and other malignancies. Gan To Kagaku Ryoho. 1985. 12:2379–2386. (English abstract).6. Tomlinson IP, Whyman A, Barrett JA, Kremer JK. Tumour marker CA15-3: possible use in the routine management of breast cancer. Eur J Cancer. 1995. 31A:899–902.7. Clinical practice guidelines for the use of tumor markers in breast and colorectal cancer. Adopted on May 17, 1996 by the American Society of Clinical Oncology. J Clin Oncol. 1996. 14:2843–2877.8. Bargmann CI, Hung MC, Weinberg RA. The neu oncogene encodes an epidermal growth factor receptor-related protein. Nature. 1986. 319:226–230.
Article9. Coussens L, Yang-Feng TL, Liao YC, Chen E, Gray A, McGrath J, et al. Tyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with neu oncogene. Science. 1985. 230:1132–1139.
Article10. Kim JW, Kim SY, Lee HS, Woo HD, Son DM, Lim CW, et al. Establishment for reference range of serum HER-2/neu in Korean healthy women. J Breast Cancer. 2006. 9:301–308.
Article11. Miki Y, Swensen J, Shattuck-Eidens D, Futreal PA, Harshman K, Tavtigian S, et al. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994. 266:66–71.
Article12. Collins N, McManus R, Wooster R, Mangion J, Seal S, Lakhani SR, et al. Consistent loss of the wild type allele in breast cancers from a family linked to the BRCA2 gene on chromosome 13q12-13. Oncogene. 1995. 10:1673–1675.13. Yoo KY, Tajima K, Kuroishi T, Hirose K, Yoshida M, Miura S, et al. Independent protective effect of lactation against breast cancer: a case-control study in Japan. Am J Epidemiol. 1992. 135:726–733.14. Harris JR, Lippman ME, Veronesi U, Willett W. Breast cancer(1). N Engl J Med. 1992. 327:319–328.15. Gunczler P, Ogris E, Maca S, Danmayr E. Tumour markers in breast cancer: on the diagnostic value of serum determinations in clinical freedom from tumour and manifest disease. Onkologie. 1989. 12:209–214. (English abstract).16. Suzuki S. Early diagnosis for bone metastases of breast cancer based on bone metabolism. Fukushima J Med Sci. 1990. 36:11–27.17. Shih C, Padhy LC, Murray M, Weinberg RA. Transforming genes of carcinomas and neuroblastomas introduced into mouse fibroblasts. Nature. 1981. 290:261–264.
Article18. Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987. 235:177–182.
Article19. Rait AS, Pirollo KF, Xiang L, Ulick D, Chang EH. Tumor-targeting, systemically delivered antisense HER-2 chemosensitizes human breast cancer xenografts irrespective of HER-2 levels. Mol Med. 2002. 8:475–486.
Article20. Storm FK, Gilchrist KW, Warner TF, Mahvi DM. Distribution of Hsp-27 and HER-2/neu in in situ and invasive ductal breast carcinomas. Ann Surg Oncol. 1995. 2:43–48.
Article21. Padhy LC, Shih C, Cowing D, Finkelstein R, Weinberg RA. Identification of a phosphoprotein specifically induced by the transforming DNA of rat neuroblastomas. Cell. 1982. 28:865–871.
Article22. Brandt-Rauf PW, Monaco R, Pincus MR. Conformation of the transmembrane domain of the epidermal growth factor receptor. J Protein Chem. 1994. 13:227–231.
Article23. Gullick WJ, Bottomley AC, Lofts FJ, Doak DG, Mulvey D, Newman R, et al. Three dimensional structure of the transmembrane region of the proto-oncogenic and oncogenic forms of the neu protein. EMBO J. 1992. 11:43–48.
Article24. Guerin M, Gabillot M, Mathieu MC, Travagli JP, Spielmann M, Andrieu N, et al. Structure and expression of c-erbB-2 and EGF receptor genes in inflammatory and non-inflammatory breast cancer: prognostic significance. Int J Cancer. 1989. 43:201–208.
Article25. Sahin AA. Biologic and clinical significance of HER-2/neu (cerbB-2) in breast cancer. Adv Anat Pathol. 2000. 7:158–166.
Article26. Krainer M, Brodowicz T, Zeillinger R, Wiltschke C, Scholten C, Seifert M, et al. Tissue expression and serum levels of HER-2/neu in patients with breast cancer. Oncology. 1997. 54:475–481.
Article27. Bofin AM, Ytterhus B, Martin C, O'Leary JJ, Hagmar BM. Detection and quantitation of HER-2 gene amplification and protein expression in breast carcinoma. Am J Clin Pathol. 2004. 122:110–119.
Article28. Tse C, Brault D, Gligorov J, Antoine M, Neumann R, Lotz JP, et al. Evaluation of the quantitative analytical methods real-time PCR for HER-2 gene quantification and ELISA of serum HER-2 protein and comparison with fluorescence in situ hybridization and immunohistochemistry for determining HER-2 status in breast cancer patients. Clin Chem. 2005. 51:1093–1101.
Article29. Kim SY, Kim TY, Kim JJ, Kim CH, Song OP, Lee MH, et al. Clinical correlation of HER-2/neu overexpression in patients with breast cancer. J Korean Breast Cancer Society. 2004. 7:244–250.
Article30. Toikanen S, Helin H, Isola J, Joensuu H. Prognostic significance of HER-2 oncoprotein expression in breast cancer: a 30-year follow-up. J Clin Oncol. 1992. 10:1044–1048.
Article31. Barnes DM, Dublin EA, Fisher CJ, Levison DA, Millis RR. Immunohistochemical detection of p53 protein in mammary carcinoma: an important new independent indicator of prognosis? Hum Pathol. 1993. 24:469–476.
Article32. Andrulis IL, Bull SB, Blackstein ME, Sutherland D, Mak C, Sidlofsky S, et al. neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. Toronto Breast Cancer Study Group. J Clin Oncol. 1998. 16:1340–1349.
Article33. Noh DY, Choe KJ, Kim JP, Park IA, Park SH, Yoo KY. DNA ploidy, S-phase activity and c-erbB-2 oncogene protein expression in breast cancer and its relationship to prognosis. J Korean Cancer Association. 1992. 24:73–81.34. Han SH, Yun IJ, Noh DY, Choe KJ, Song SY, Chi JG. Abnormal expression of four novel molecular markers represents a highly aggressive phenotype in breast cancer. Immunohistochemical assay of p53, nm23, erbB-2, and cathepsin D protein. J Surg Oncol. 1997. 65:22–27.
Article35. Gsaparini G, Bevilacqua P, Pozza F, Meli S, Boracchi P, Marubini E, et al. Value of epidermal growth factor receptor status compared with growth fraction and other factors for prognosis in early breast cancer. Br J Cancer. 1992. 66:970–976.
Article36. Osaki A, Toi M, Yamada H, Kawami H, Kuroi K, Toge T. Prognostic significance of co-expression of c-erbB-2 oncoprotein and epidermal growth factor receptor in breast cancer patients. Am J Surg. 1992. 164:323–326.
Article37. Park W, Paik NS, Kim YK, Moon NM, Lee JI, Choi DW, et al. The expression of c-erbB-2 oncoprotein and epidermal growth factor receptor (EGFR) in breast cancer patients in Korea. J Korean Cancer Association. 1994. 26:901–911.38. Luftner D, Cheli C, Mickelson K, Sampson E, Possinger K. ADVIA Centaur HER-2/neu shows value in monitoring patients with metastatic breast cancer. Int J Biol Markers. 2004. 19:175–182.
Article39. Luftner D, Luke C, Possinger K. Serum HER-2/neu in the management of breast cancer patients. Clin Biochem. 2003. 36:233–240.
Article40. Ali SM, Leitzel K, Chinchilli VM, Engle L, Demers L, Harvey HA, et al. Relationship of serum HER-2/neu and serum CA15-3 in patients with metastatic breast cancer. Clin Chem. 2002. 48:1314–1320.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation between the Her-2/neu Status as Determined by Immunohistochemical Analysis and the Serum Her-2/neu Concentration as Determined by the Use of ADVIA Cencaur(R) Automated Immunoassay in Breast Cancer Patients
- Serum HER2 as a Response Indicator to Various Chemotherapeutic Agents in Tissue HER2 Positive Metastatic Breast Cancer
- Clinical significance of serum CA15-3 as a prognostic parameter during follow-up periods in patients with breast cancer
- Numerical aberrations of chromosome 17 and her2/neu gene amplification, her2/neu and p 53 protein expression in breast cancer
- Clinical Value of CEA, CA15-3 and TPS in Breast Cancer