Ann Surg Treat Res.
2016 Feb;90(2):57-63. 10.4174/astr.2016.90.2.57.
Clinical significance of serum CA15-3 as a prognostic parameter during follow-up periods in patients with breast cancer
- Affiliations
-
- 1Division of Breast Surgery, Department of Surgery, Kosin University Gospel Hospital, Busan, Korea. lovebreast@naver.com
- KMID: 2166823
- DOI: http://doi.org/10.4174/astr.2016.90.2.57
Abstract
- PURPOSE
To assess the relationship between the kinetics of the serum CA15-3 level and the five-year disease-free survival rate of breast cancer patients.
METHODS
The subjects of this study, 297 women who were diagnosed with breast cancer, were the subset of patients operated on at Kosin University Gospel Hospital from January 2008 to December 2010. We evaluated the change of serum CA15-3 levels during outpatient follow-up period. The changing patterns of serum CA15-3 level were divided into 5 categories; surge without decline, surge with incidental decline, decline without surge, decline with incidental surge, and no change. Clinicopathologic factors were evaluated for each group.
RESULTS
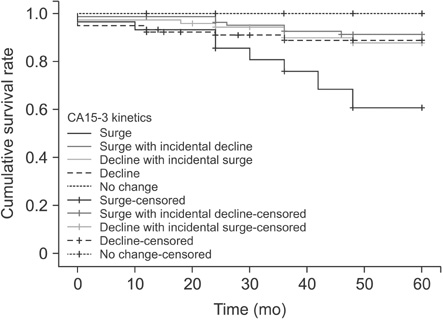
The number of patients in surge without decline, surge with incidental decline, decline without surge, decline with incidental surge, and no changes groups were 30 (10.1%), 85 (28.6%), 80 (26.9%), 73 (24.6%), and 29 (9.7%), respectively. The clinicopathologic characteristics were not significantly different among these groups. The log rank test found that 5-year disease-free survival rate according to the kinetics of serum CA15-3 levels were significant (P = 0.004) particularly for the surge without decline group.
CONCLUSION
According to the findings of this study, the surge without incidental decline pattern of serum CA15-3 levels during the follow-up period is associated with poor prognosis. Significant association was found among changing patterns of serum CA15-3 levels and breast cancer recurrence rate.
Keyword
MeSH Terms
Figure
Reference
-
1. Shao Y, Sun X, He Y, Liu C, Liu H. Elevated levels of serum tumor markers CEA and CA15-3 are prognostic parameters for different molecular subtypes of breast cancer. PLoS One. 2015; 10:e0133830.2. Wu SG, He ZY, Zhou J, Sun JY, Li FY, Lin Q, et al. Serum levels of CEA and CA15-3 in different molecular subtypes and prognostic value in Chinese breast cancer. Breast. 2014; 23:88–93.3. Wang G, Qin Y, Zhang J, Zhao J, Liang Y, Zhang Z, et al. Nipple discharge of CA15-3, CA125, CEA and TSGF as a new biomarker panel for breast cancer. Int J Mol Sci. 2014; 15:9546–9565.4. Zhang SJ, Hu Y, Qian HL, Jiao SC, Liu ZF, Tao HT, et al. Expression and significance of ER, PR, VEGF, CA15-3, CA125 and CEA in judging the prognosis of breast cancer. Asian Pac J Cancer Prev. 2013; 14:3937–3940.5. Thriveni K, Deshmane V, Ramaswamy G, Krishnamoorthy L. Diagnostic significance of CA15-3 with combination of HER-2/neu values at 85th percentiles in breast cancer. Indian J Clin Biochem. 2013; 28:136–140.6. Tarhan MO, Gonel A, Kucukzeybek Y, Erten C, Cuhadar S, Yigit SC, et al. Prognostic significance of circulating tumor cells and serum CA15-3 levels in metastatic breast cancer, single center experience, preliminary results. Asian Pac J Cancer Prev. 2013; 14:1725–1729.7. Nisman B, Maimon O, Allweis T, Kadouri L, Maly B, Hamburger T, et al. The prognostic significance of LIAISON(R) CA15-3 assay in primary breast cancer. Anticancer Res. 2013; 33:293–299.8. Kim HS, Park YH, Park MJ, Chang MH, Jun HJ, Kim KH, et al. Clinical significance of a serum CA15-3 surge and the usefulness of CA15-3 kinetics in monitoring chemotherapy response in patients with metastatic breast cancer. Breast Cancer Res Treat. 2009; 118:89–97.9. Hiramoto Y, Tamada R, Sugimachi K, Nomura Y. The clinical value of a cancer antigen CA15-3 as a tumor associated antigen in breast carcinoma. Rinsho Byori. 1986; 34:1049–1052.10. Tomlinson IP, Whyman A, Barrett JA, Kremer JK. Tumour marker CA15-3: possible uses in the routine management of breast cancer. Eur J Cancer. 1995; 31A:899–902.11. Di Gioia D, Heinemann V, Nagel D, Untch M, Kahlert S, Bauerfeind I, et al. Kinetics of CEA and CA15-3 correlate with treatment response in patients undergoing chemotherapy for metastatic breast cancer (MBC). Tumour Biol. 2011; 32:777–785.12. Lauro S, Trasatti L, Bordin F, Lanzetta G, Bria E, Gelibter A, et al. Comparison of CEA, MCA, CA 15-3 and CA 27-29 in follow-up and monitoring therapeutic response in breast cancer patients. Anticancer Res. 1999; 19(4C):3511–3515.13. Kikuchi K, Uematsu Y, Takada Y, Kurihara E, Suito T, Fujisaki M, et al. Evaluation of tumor marker CA15-3 in breast cancer. Gan To Kagaku Ryoho. 1987; 14:3095–3100.14. Lufter D, Richter A, Gunther S, Flath B, Akrivakis C, Geppert R, et al. A comparison of bone-related biomarkers and CA27.29 to assess response to treatment of osseous metastatic breast cancer. Anticancer Res. 2000; 20(6D):5099–5105.15. Tondini C, Hayes DF, Gelman R, Henderson IC, Kufe DW. Comparison of CA15-3 and carcinoembryonic antigen in monitoring the clinical course of patients with metastatic breast cancer. Cancer Res. 1988; 48:4107–4112.16. Loprinzi CL, Tormey DC, Rasmussen P, Falkson G, Davis TE, Falkson HC, et al. Prospective evaluation of carcinoembryonic antigen levels and alternating chemotherapeutic regimens in metastatic breast cancer. J Clin Oncol. 1986; 4:46–56.17. Agha-Hosseini F, Mirzaii-Dizgah I, Rahimi A. Correlation of serum and salivary CA15-3 levels in patients with breast cancer. Med Oral Patol Oral Cir Bucal. 2009; 14:e521–e524.18. Ali HQ, Mahdi NK, Al-Jowher MH. The value of CA15-3 in diagnosis, prognosis and treatment response in women with breast cancer. J Pak Med Assoc. 2013; 63:1138–1141.19. Berruti A, Tampellini M, Torta M, Buniva T, Gorzegno G, Dogliotti L. Prognostic value in predicting overall survival of two mucinous markers: CA 15-3 and CA 125 in breast cancer patients at first relapse of disease. Eur J Cancer. 1994; 30A:2082–2084.20. Kobayashi S, Iwase H, Karamatsu S, Matsuo K, Masaoka A, Miyagawa T. The clinical value of serum CA15-3 assay postoperatively in breast cancer patients. Jpn J Surg. 1989; 19:278–282.21. Devine PL, Duroux MA, Quin RJ, McGuckin MA, Joy GJ, Ward BG, et al. CA15-3, CASA, MSA, and TPS as diagnostic serum markers in breast cancer. Breast Cancer Res Treat. 1995; 34:245–251.22. Pons-Anicet DM, Krebs BP, Mira R, Namer M. Value of CA 15:3 in the follow-up of breast cancer patients. Br J Cancer. 1987; 55:567–569.23. Bliss P, Fisken J, Roulsten J, Leonard RC. An assessment of the clinical usefulness of two serum markers, CA15 3 and HMFG 2 in localized and metastatic breast cancer. Dis Markers. 1993; 11:45–48.24. Brooks M. Breast cancer screening and biomarkers. Methods Mol Biol. 2009; 472:307–321.25. Daly L, Ferguson J, Cram GP Jr, Hars V, George SL, McCarty KS Jr, et al. Comparison of a novel assay for breast cancer mucin to CA15-3 and carcinoembryonic antigen. J Clin Oncol. 1992; 10:1057–1065.26. Darwish IA, Wani TA, Khalil NY, Blake DA. Novel automated flow-based immunosensor for real-time measurement of the breast cancer biomarker CA15-3 in serum. Talanta. 2012; 97:499–504.27. Hayes DF, Zurawski VR Jr, Kufe DW. Comparison of circulating CA15-3 and carcinoembryonic antigen levels in patients with breast cancer. J Clin Oncol. 1986; 4:1542–1550.28. Keyhani M, Nasizadeh S, Dehghannejad A. Serum CA15-3 measurement in breast cancer patients before and after mastectomy. Arch Iranian Med. 2005; 8:263–266.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Value of CEA, CA15-3 and TPS in Breast Cancer
- Clinical Value of CEA, CA15-3 and TPS in Breast Cancer
- Serum HER2 as a Response Indicator to Various Chemotherapeutic Agents in Tissue HER2 Positive Metastatic Breast Cancer
- Prognostic Value of the Preoperative CEA, CA15-3 and TPS Serum Levels in Patients with Breast Cancer
- The Correlation of Serum HER-2/neu and CA15-3 in Patients with Metastatic Breast Cancer