Korean J Physiol Pharmacol.
2015 Sep;19(5):401-411. 10.4196/kjpp.2015.19.5.401.
Amelioration of Bleomycin-induced Pulmonary Fibrosis of Rats by an Aldose Reductase Inhibitor, Epalrestat
- Affiliations
-
- 1Department of Pharmacology, Wannan Medical College, Wuhu 241002, China. wnmclixianwei69@163.com
- KMID: 2285584
- DOI: http://doi.org/10.4196/kjpp.2015.19.5.401
Abstract
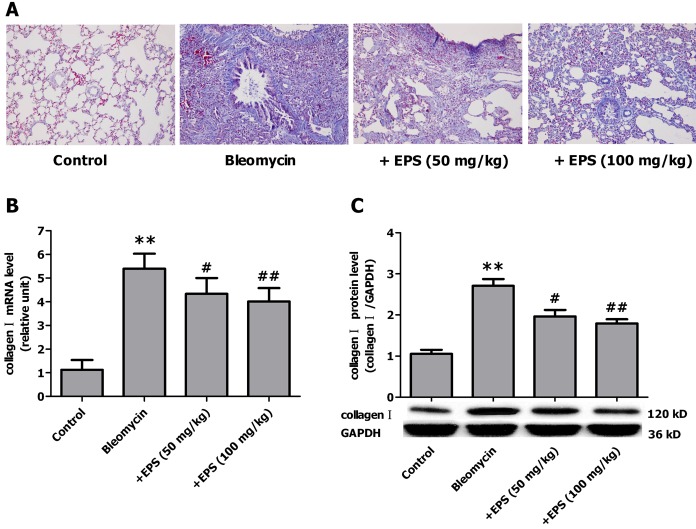
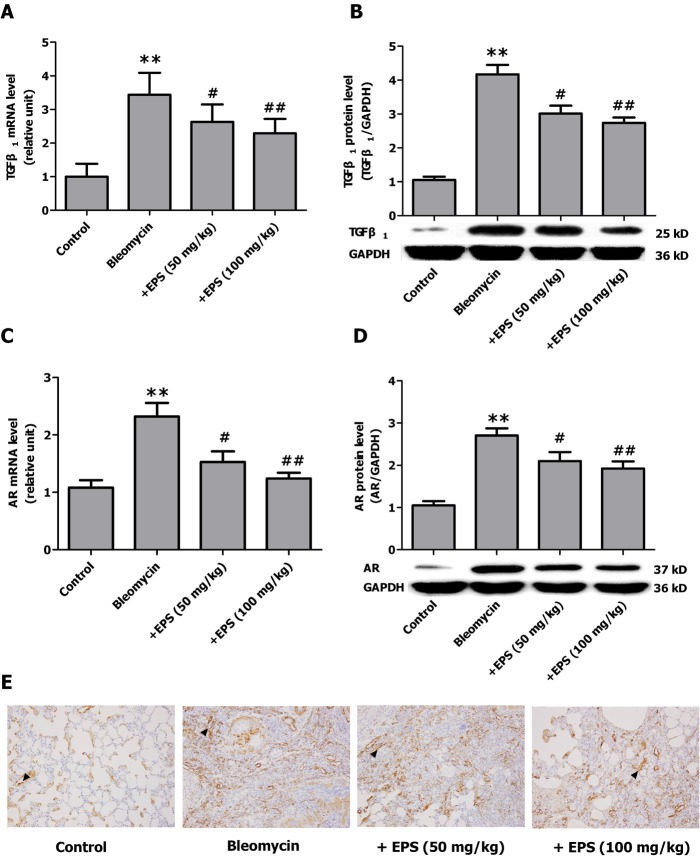
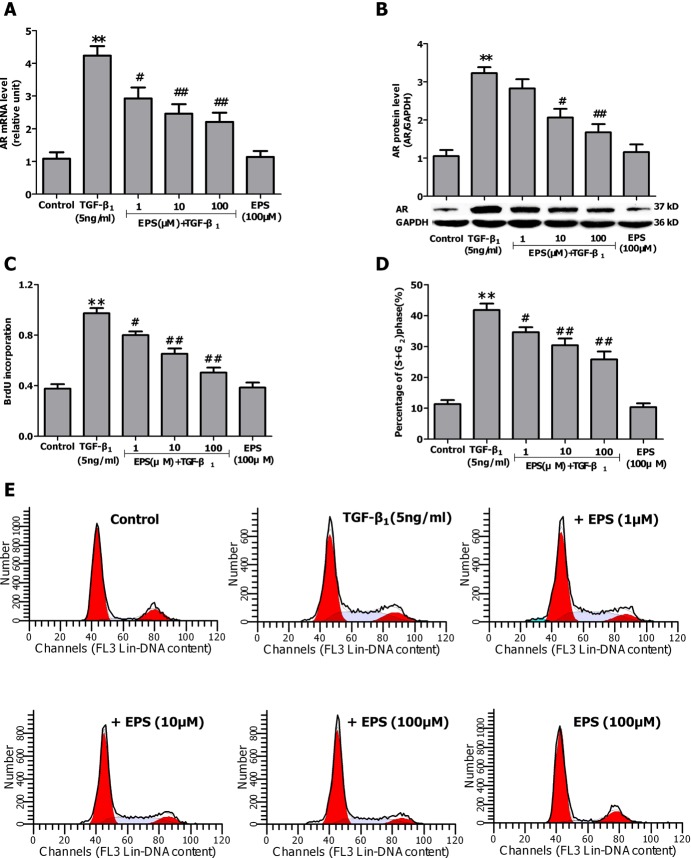
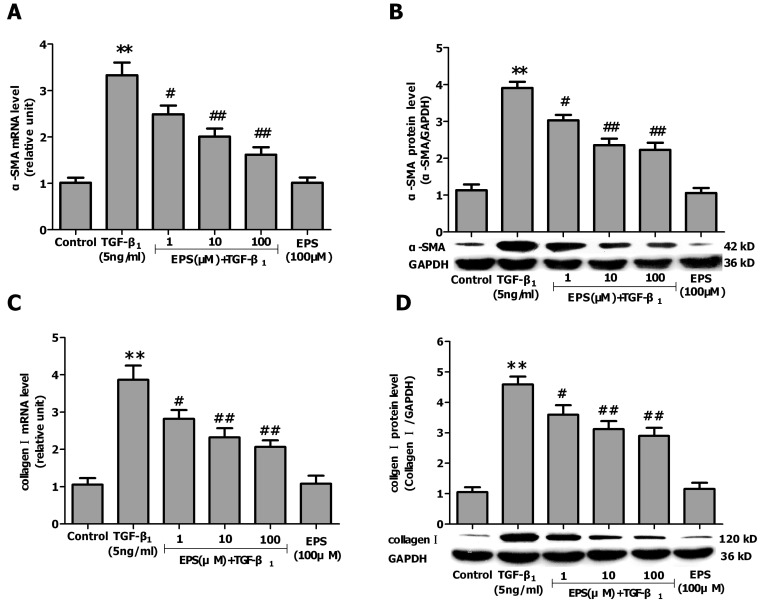
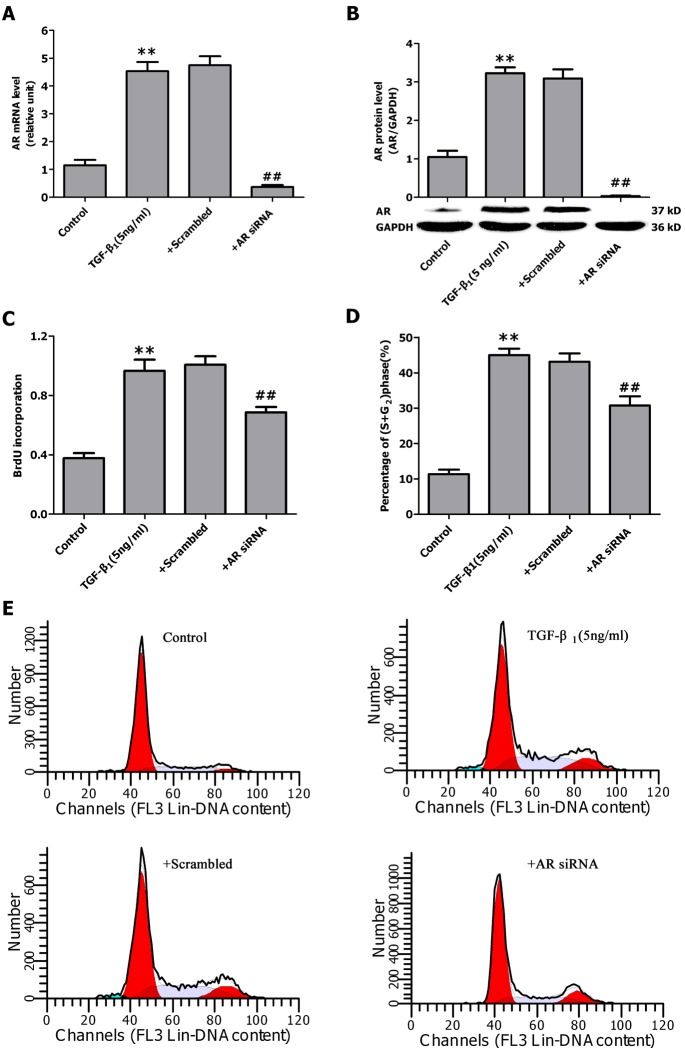
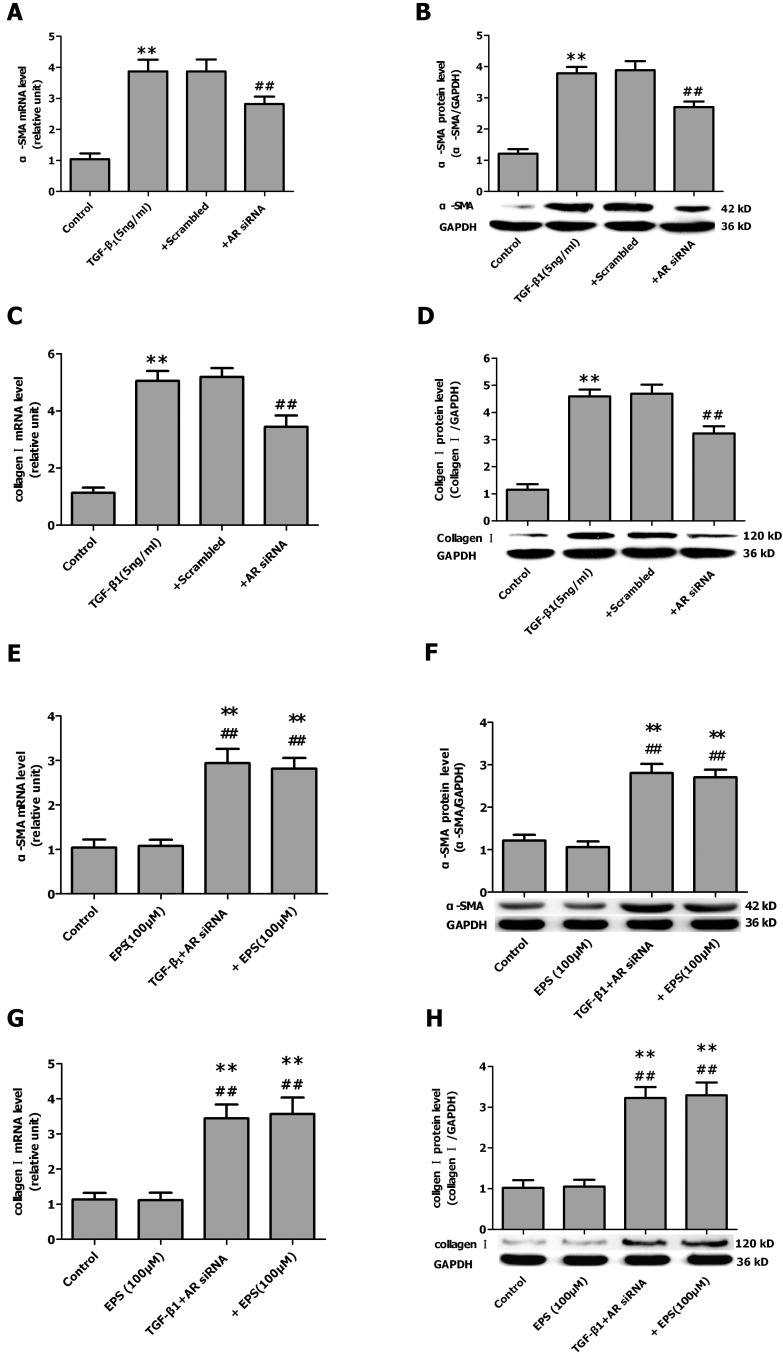
- Aldose reductase (AR) is known to play a crucial role in the mediation of diabetic and cardiovascular complications. Recently, several studies have demonstrated that allergen-induced airway remodeling and ovalbumin-induced asthma is mediated by AR. Epalrestat is an aldose reductase inhibitor that is currently available for the treatment of diabetic neuropathy. Whether AR is involved in pathogenesis of pulmonary fibrosis and whether epalrestat attenuates pulmonary fibrosis remains unknown. Pulmonary fibrosis was induced by intratracheal instillation of bleomycin (5 mg/kg) in rats. Primary pulmonary fibroblasts were cultured to investigate the proliferation by BrdU incorporation method and flow cytometry. The expression of AR, TGF-beta1, alpha-SMA and collagen I was analyzed by immunohistochemisty, real-time PCR or western blot. In vivo, epalrestat treatment significantly ameliorated the bleomycin-mediated histological fibrosis alterations and blocked collagen deposition concomitantly with reversing bleomycin-induced expression up-regulation of TGF-beta1, AR, alpha-SMA and collagen I (both mRNA and protein). In vitro, epalrestat remarkably attenuated proliferation of pulmonary fibroblasts and expression of alpha-SMA and collagen I induced by TGF-beta1, and this inhibitory effect of epalrestat was accompanied by inhibiting AR expression. Knockdown of AR gene expression reversed TGF-beta1-induced proliferation of fibroblasts, up-regulation of alpha-SMA and collagen I expression. These findings suggest that AR plays an important role in bleomycin-induced pulmonary fibrosis, and epalrestat inhibited the progression of bleomycin-induced pulmonary fibrosis is mediated via inhibiting of AR expression.
MeSH Terms
-
Airway Remodeling
Aldehyde Reductase*
Animals
Asthma
Bleomycin
Blotting, Western
Bromodeoxyuridine
Collagen
Diabetic Neuropathies
Fibroblasts
Fibrosis
Flow Cytometry
Gene Expression
Negotiating
Pulmonary Fibrosis*
Rats*
Real-Time Polymerase Chain Reaction
RNA, Messenger
Transforming Growth Factor beta1
Up-Regulation
Aldehyde Reductase
Bleomycin
Bromodeoxyuridine
Collagen
RNA, Messenger
Transforming Growth Factor beta1
Figure
Cited by 1 articles
-
Inhibitory effects of 2,6-di-
tert -butyl-4-hydroxymethylphenol on asthmatic responses to ovalbumin challenge in conscious guinea pigs
Seul-Yong Jeong, Ji-Yun Lee
Korean J Physiol Pharmacol. 2018;22(1):81-89. doi: 10.4196/kjpp.2018.22.1.81.
Reference
-
1. Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001; 345:517–525. PMID: 11519507.
Article2. O'Connell OJ, Kennedy MP, Henry MT. Idiopathic pulmonary fibrosis: treatment update. Adv Ther. 2011; 28:986–999. PMID: 21975927.3. Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011; 208:1339–1350. PMID: 21727191.
Article4. Scotton CJ, Chambers RC. Molecular targets in pulmonary fibrosis: the myofibroblast in focus. Chest. 2007; 132:1311–1321. PMID: 17934117.5. Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007; 170:1807–1816. PMID: 17525249.6. Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002; 122(6 Suppl):286S–289S. PMID: 12475801.
Article7. Anil Kumar P, Bhanuprakash Reddy G. Focus on molecules: aldose reductase. Exp Eye Res. 2007; 85:739–740. PMID: 16997295.
Article8. Vedantham S, Ananthakrishnan R, Schmidt AM, Ramasamy R. Aldose reductase, oxidative stress and diabetic cardiovascular complications. Cardiovasc Hematol Agents Med Chem. 2012; 10:234–240. PMID: 22632267.
Article9. Ravindranath TM, Mong PY, Ananthakrishnan R, Li Q, Quadri N, Schmidt AM, Ramasamy R, Wang Q. Novel role for aldose reductase in mediating acute inflammatory responses in the lung. J Immunol. 2009; 183:8128–8137. PMID: 20007578.
Article10. Takahashi K, Mizukami H, Kamata K, Inaba W, Kato N, Hibi C, Yagihashi S. Amelioration of acute kidney injury in lipopolysaccharide-induced systemic inflammatory response syndrome by an aldose reductase inhibitor, fidarestat. PLoS One. 2012; 7:e30134. PMID: 22253906.
Article11. Tammali R, Reddy AB, Saxena A, Rychahou PG, Evers BM, Qiu S, Awasthi S, Ramana KV, Srivastava SK. Inhibition of aldose reductase prevents colon cancer metastasis. Carcinogenesis. 2011; 32:1259–1267. PMID: 21642355.
Article12. Srivastava SK, Yadav UC, Reddy AB, Saxena A, Tammali R, Shoeb M, Ansari NH, Bhatnagar A, Petrash MJ, Srivastava S, Ramana KV. Aldose reductase inhibition suppresses oxidative stress-induced inflammatory disorders. Chem Biol Interact. 2011; 191:330–338. PMID: 21354119.
Article13. Jiang T, Che Q, Lin Y, Li H, Zhang N. Aldose reductase regulates TGF-beta1-induced production of fibronectin and type IV collagen in cultured rat mesangial cells. Nephrology (Carlton). 2006; 11:105–112. PMID: 16669970.
Article14. Huang P, Zhang Y, Jiang T, Zeng W, Zhang N. Aldose reductase is a potent regulator of TGF-β1 induced expression of fibronectin in human mesangial cells. Mol Biol Rep. 2010; 37:3097–3103. PMID: 19847669.
Article15. Zou L, Wang W, Xu Z, Zhang N, Jiang T. Aldose reductase regulates platelet-derived growth factor-induced proliferation through mediating cell cycle progression in rat mesangial cells. Int J Mol Med. 2012; 30:409–416. PMID: 22614447.
Article16. Zhang Y, Huang P, Jiang T, Zhao J, Zhang N. Role of aldose reductase in TGF-beta1-induced fibronectin synthesis in human mesangial cells. Mol Biol Rep. 2010; 37:2735–2742. PMID: 19760097.17. Yadav UC, Ramana KV, Aguilera-Aguirre L, Boldogh I, Boulares HA, Srivastava SK. Inhibition of aldose reductase prevents experimental allergic airway inflammation in mice. PLoS One. 2009; 4:e6535. PMID: 19657391.
Article18. Yadav UC, Naura AS, Aguilera-Aguirre L, Ramana KV, Boldogh I, Sur S, Boulares HA, Srivastava SK. Aldose reductase inhibition suppresses the expression of Th2 cytokines and airway inflammation in ovalbumin-induced asthma in mice. J Immunol. 2009; 183:4723–4732. PMID: 19752229.
Article19. Yadav UC, Aguilera-Aguirre L, Ramana KV, Boldogh I, Srivastava SK. Aldose reductase inhibition prevents metaplasia of airway epithelial cells. PLoS One. 2010; 5:e14440. PMID: 21203431.
Article20. Yadav UC, Naura AS, Aguilera-Aguirre L, Boldogh I, Boulares HA, Calhoun WJ, Ramana KV, Srivastava SK. Aldose reductase inhibition prevents allergic airway remodeling through PI3K/AKT/GSK3β pathway in mice. PLoS One. 2013; 8:e57442. PMID: 23460857.
Article21. Steiling K, Lenburg ME, Spira A. Airway gene expression in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009; 6:697–700. PMID: 20008878.
Article22. El-Kabbani O, Podjarny A. Selectivity determinants of the aldose and aldehyde reductase inhibitor-binding sites. Cell Mol Life Sci. 2007; 64:1970–1978. PMID: 17497245.
Article23. Ramirez MA, Borja NL. Epalrestat: an aldose reductase inhibitor for the treatment of diabetic neuropathy. Pharmacotherapy. 2008; 28:646–655. PMID: 18447661.
Article24. Steele JW, Faulds D, Goa KL. Epalrestat: a review of its pharmacology, and therapeutic potential in late-onset complications of diabetes mellitus. Drugs Aging. 1993; 3:532–555. PMID: 8312678.25. Yasunari K, Kohno M, Kano H, Minami M, Yoshikawa J. Aldose reductase inhibitor improves insulin-mediated glucose uptake and prevents migration of human coronary artery smooth muscle cells induced by high glucose. Hypertension. 2000; 35:1092–1098. PMID: 10818070.
Article26. Li ZY, Deng XL, Huang WH, Li L, Li H, Jing X, Tian YY, Lv PY, Yang TL, Zhou HH, Ouyang DS. Lignans from the bark of Eucommia ulmoides inhibited Ang II-stimulated extracellular matrix biosynthesis in mesangial cells. Chin Med. 2014; 9:8. PMID: 24524265.
Article27. Gu J, Wang JJ, Yan J, Cui CF, Wu WH, Li L, Wang ZS, Yu M, Gao N, Liu L, Ouyang DS. Effects of lignans extracted from Eucommia ulmoides and aldose reductase inhibitor epalrestat on hypertensive vascular remodeling. J Ethnopharmacol. 2011; 133:6–13. PMID: 20817083.
Article28. Li L, Yan J, Hu K, Gu J, Wang JJ, Deng XL, Li H, Jing X, Li ZY, Ye QF, Ouyang DS. Protective effects of Eucommia lignans against hypertensive renal injury by inhibiting expression of aldose reductase. J Ethnopharmacol. 2012; 139:454–461. PMID: 22138658.
Article29. Li XW, Wu YH, Li XH, Li D, Du J, Hu CP, Li YJ. Role of eukaryotic translation initiation factor 3a in bleomycin-induced pulmonary fibrosis. Eur J Pharmacol. 2015; 749:89–97. PMID: 25592322.
Article30. Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988; 41:467–470. PMID: 3366935.
Article31. Zhang H, Liu X, Chen S, Wu J, Ye X, Xu L, Chen H, Zhang D, Tan R, Wang Y. Tectorigenin inhibits the in vitro proliferation and enhances miR-338* expression of pulmonary fibroblasts in rats with idiopathic pulmonary fibrosis. J Ethnopharmacol. 2010; 131:165–173. PMID: 20600766.32. Gu L, Zhu YJ, Yang X, Guo ZJ, Xu WB, Tian XL. Effect of TGF-beta/Smad signaling pathway on lung myofibroblast differentiation. Acta Pharmacol Sin. 2007; 28:382–391. PMID: 17303001.33. Jiang HD, Guan HS. MS80, a novel sulfated oligosaccharide, inhibits pulmonary fibrosis by targeting TGF-beta1 both in vitro and in vivo. Acta Pharmacol Sin. 2009; 30:973–979. PMID: 19543300.34. Kisseleva T, Brenner DA. Mechanisms of fibrogenesis. Exp Biol Med (Maywood). 2008; 233:109–122. PMID: 18222966.
Article35. Thavarajah K, Wu P, Rhew EJ, Yeldandi AK, Kamp DW. Pulmonary complications of tumor necrosis factor-targeted therapy. Respir Med. 2009; 103:661–669. PMID: 19201589.
Article36. Khalil N, Parekh TV, O'Connor R, Antman N, Kepron W, Yehaulaeshet T, Xu YD, Gold LI. Regulation of the effects of TGF-beta 1 by activation of latent TGF-beta 1 and differential expression of TGF-beta receptors (T beta R-I and T beta R-II) in idiopathic pulmonary fibrosis. Thorax. 2001; 56:907–915. PMID: 11713352.37. Coker RK, Laurent GJ, Jeffery PK, du Bois RM, Black CM, McAnulty RJ. Localisation of transforming growth factor beta1 and beta3 mRNA transcripts in normal and fibrotic human lung. Thorax. 2001; 56:549–556. PMID: 11413354.
Article38. Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997; 100:768–776. PMID: 9259574.
Article39. Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005; 26:380–392. PMID: 15814847.
Article40. Hotta N, Akanuma Y, Kawamori R, Matsuoka K, Oka Y, Shichiri M, Toyota T, Nakashima M, Yoshimura I, Sakamoto N, Shigeta Y. Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy: the 3-year, multicenter, comparative Aldose Reductase Inhibitor-Diabetes Complications Trial. Diabetes Care. 2006; 29:1538–1544. PMID: 16801576.41. Hotta N, Kawamori R, Fukuda M, Shigeta Y. Aldose Reductase Inhibitor-Diabetes Complications Trial Study Group. Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on progression of diabetic neuropathy and other microvascular complications: multivariate epidemiological analysis based on patient background factors and severity of diabetic neuropathy. Diabet Med. 2012; 29:1529–1533. PMID: 22507139.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Esculetin, a Coumarin Derivative, Inhibits Aldose Reductase Activity in vitro and Cataractogenesis in Galactose-Fed Rats
- Scanning electron microscopic study of capillary change in bleomycin-induced pulmonary fibrosis
- Increased aldose reductase activity of cultured endothelial cell inhigh glucose media and reserve by epalrestat
- An Ultrastructural Study of Bleomycin-Induced Interstitial Pulmonary Fibrosis in the Rat
- Inhibitory effect of nordihydroguiaretic acid on bleomycin-induced pulmonary fibrosis in rat