Korean J Physiol Pharmacol.
2015 Mar;19(2):151-158. 10.4196/kjpp.2015.19.2.151.
The Effect of Polyphenols Isolated from Cynanchi wilfordii Radix with Anti-inflammatory, Antioxidant, and Anti-bacterial Activity
- Affiliations
-
- 1Department of Microbiology, Chung-Ang University College of Medicine, Seoul 156-756, Korea. kimwy@cau.ac.kr
- 2Asia Pacific International School, Seoul 139-852, Korea.
- 3Department of Pharmacology, College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea.
- KMID: 2285567
- DOI: http://doi.org/10.4196/kjpp.2015.19.2.151
Abstract
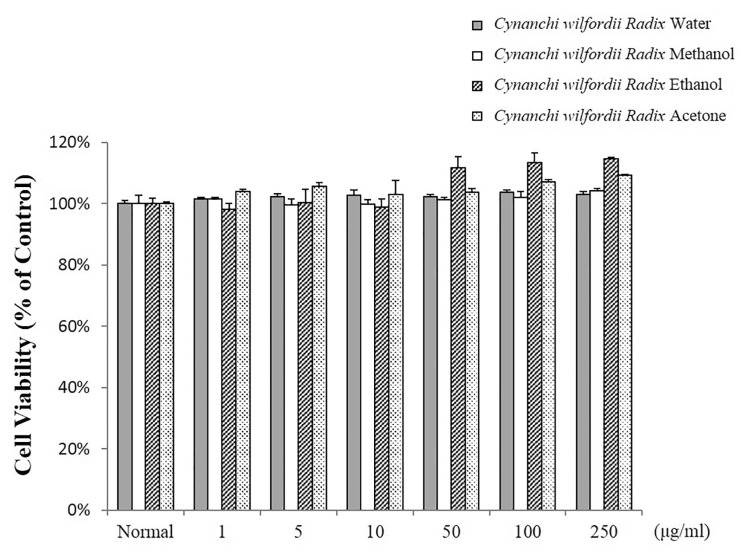
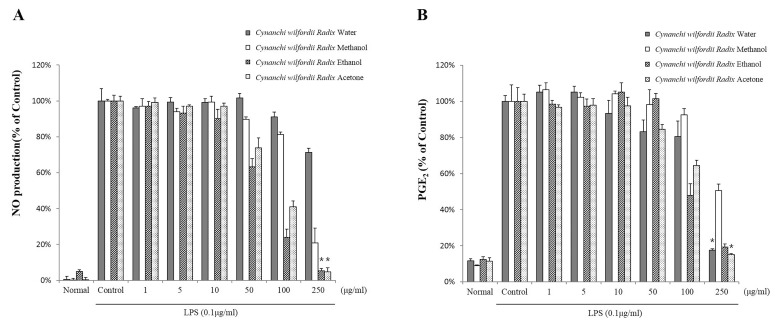
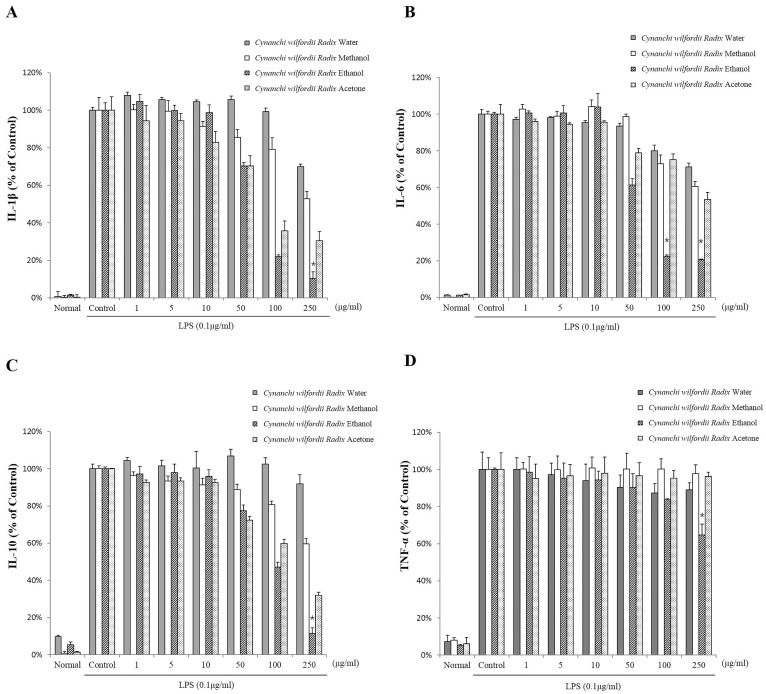
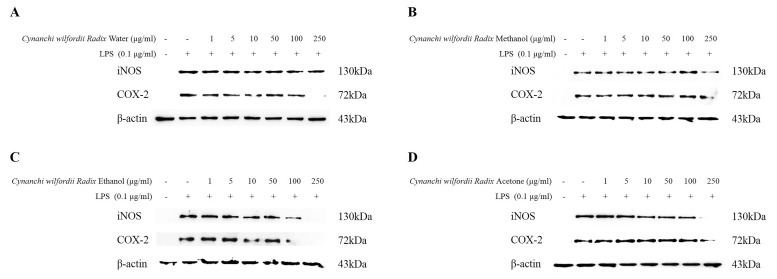
- Recently, Cynanchi wilfordii Radix has gained wide use in Asian countries as a functional food effective for relieving fatigue, osteoporosis, and constipation, particularly in menopausal disorders. However, its anti-inflammatory and anti-microbial activities have not been explored in detail to date. The anti-inflammatory, antioxidant, and anti-bacterial properties of the Cynanchi wilfordii Radix extracts obtained with water, methanol, ethanol, and acetone were compared. All 4 polyphenol-containing extracts exhibited anti-inflammatory and antioxidant effects. The ethanol extract was found to elicit the most potent reduction of nitric oxide (NO), prostaglandin E2 (PGE2), and cytokine (IL-1beta, IL-6, IL-10, and TNF-alpha) levels, as well as inhibit the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in a concentration-dependent manner. The evaluation of antioxidant activity also revealed the ethanol extract to have the highest free radical scavenging activity, measured as 85.3+/-0.4%, which is equivalent to 99.9% of the activity of alpha -tocopherol. In the assessment of anti-bacterial activity, only ethanol extract was found to inhibit the growth of the Bacillus species Bacillus cereus and Bacillus anthracis. These results show that polyphenols of Cynanchi wilfordii Radix have anti-inflammatory, antioxidant, and anti-bacterial properties that can be exploited and further improved for use as a supplementary functional food, in cosmetics, and for pharmaceutical purposes.
Keyword
MeSH Terms
-
Acetone
Antioxidants
Asian Continental Ancestry Group
Bacillus
Bacillus anthracis
Bacillus cereus
Constipation
Cyclooxygenase 2
Dinoprostone
Ethanol
Fatigue
Functional Food
Humans
Interleukin-10
Interleukin-6
Methanol
Nitric Oxide
Nitric Oxide Synthase Type II
Osteoporosis
Polyphenols*
Water
Acetone
Antioxidants
Cyclooxygenase 2
Dinoprostone
Ethanol
Interleukin-10
Interleukin-6
Methanol
Nitric Oxide
Nitric Oxide Synthase Type II
Polyphenols
Water
Figure
Reference
-
1. Lamidi M, DiGiorgio C, Delmas F, Favel A, Eyele Mve-Mba C, Rondi ML, Ollivier E, Nze-Ekekang L, Balansard G. In vitro cytotoxic, antileishmanial and antifungal activities of ethnopharmacologically selected Gabonese plants. J Ethnopharmacol. 2005; 102:185–190. PMID: 16046090.
Article2. Han DS. Pharmacoligical studies on Ha-Su-O (Polygoni Radix). Korean J Pharmacogn. 1973; 4:83–94.3. Deepak D, Srivastav S, Khare A. Pregnane glycosides. Fortschr Chem Org Naturst. 1997; 71:169–325. PMID: 9250024.4. Kaneda N, Chai H, Pezzuto JM, Kinghorn AD, Farnsworth NR, Tuchinda P, Udchachon J, Santisuk T, Reutrakul V. Cytotoxic activity of cardenolides from Beaumontia brevituba stems. Planta Med. 1992; 58:429–431. PMID: 1470666.5. Seo YW, Kim HH, Ko H. Effect of aqueous extract of polygoni multiflori radix on hypertension and arterial contraction in animal models. Korean J Orient Physiol Pathol. 2008; 22:593–599.6. Shin MK. A Comparative study on the effects of polygoni radix and cynanchi radix on rat livers intoxicated with carbon tetrachloride. Korean J Pharmacogn. 1985; 16:81–92.7. Lawrence T, Willoughby DA, Gilroy DW. Anti-inflammatory lipid mediators and insights into the resolution of inflammation. Nat Rev Immunol. 2002; 2:787–795. PMID: 12360216.
Article8. Kaplanski G, Marin V, Montero-Julian F, Mantovani A, Farnarier C. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 2003; 24:25–29. PMID: 12495721.
Article9. Boscá L, Zeini M, Través PG, Hortelano S. Nitric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate. Toxicology. 2005; 208:249–258. PMID: 15691589.
Article10. Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005; 23:787–819. PMID: 15771586.
Article11. Halliwell B. Protection against tissue damage in vivo by desferrioxamine: what is its mechanism of action? Free Radic Biol Med. 1989; 7:645–651. PMID: 2695408.
Article12. Morrissey PA, O'Brien NM. Dietary antioxidants in health and disease. Int Dairy J. 1998; 8:463–472.
Article13. Ehling-Schulz M, Fricker M, Scherer S. Bacillus cereus, the causative agent of an emetic type of food-borne illness. Mol Nutr Food Res. 2004; 48:479–487. PMID: 15538709.
Article14. Hilliard NJ, Schelonka RL, Waites KB. Bacillus cereus bacteremia in a preterm neonate. J Clin Microbiol. 2003; 41:3441–3444. PMID: 12843116.15. Oh BK, Kim YK, Park KW, Lee WH, Choi JW. Surface plasmon resonance immunosensor for the detection of Salmonella typhimurium. Biosens Bioelectron. 2004; 19:1497–1504. PMID: 15093222.
Article16. Paton JC, Paton AW. Pathogenesis and diagnosis of Shiga toxin-producing Escherichia coli infections. Clin Microbiol Rev. 1998; 11:450–479. PMID: 9665978.17. Singh N, Singh RK, Bhunia AK, Stroshine RL. Efficacy of chlorine dioxide, ozone, and thyme essential oil or a sequential washing in killing escherichia coli O157: H7 on lettuce and baby carrots. Lebensm-Wiss Techno. 2002; 35:720–729.18. Ghelardi E, Celandroni F, Salvetti S, Barsotti C, Baggiani A, Senesi S. Identification and characterization of toxigenic Bacillus cereus isolates responsible for two food-poisoning outbreaks. FEMS Microbiol Lett. 2002; 208:129–134. PMID: 11934506.19. Saile E, Koehler TM. Control of anthrax toxin gene expression by the transition state regulator abrB. J Bacteriol. 2002; 184:370–380. PMID: 11751813.20. Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Hauer J, McDade J, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Tonat K. Working Group on Civilian Biodefense. Anthrax as a biological weapon: medical and public health management. JAMA. 1999; 281:1735–1745. PMID: 10328075.21. Folin O, Denis W. On phosphotungastic-phosphomolybdic compounds as color ragents. J Biol Chem. 1912; 12:239–243.22. Hatano T, Kagawa H, Yasuhara T, Okuda T. Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effects. Chem Pharm Bull (Tokyo). 1988; 36:2090–2097. PMID: 3240445.
Article23. LCCLS. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard-eighth edition. Clinical and Laboratory Standards Institute;2009.24. Vinson JA, Hao Y, Su XH, Zubik L. Phenol antioxidant quantity and quality in foods: Vegetables. J Agr Food Chem. 1998; 46:3630–3634.
Article25. Vinson JA, Su X, Zubik L, Bose P. Phenol antioxidant quantity and quality in foods: fruits. J Agric Food Chem. 2001; 49:5315–5321. PMID: 11714322.
Article26. Vinson JA, Teufel K, Wu N. Red wine, dealcoholized red wine, and especially grape juice, inhibit atherosclerosis in a hamster model. Atherosclerosis. 2001; 156:67–72. PMID: 11368998.
Article27. Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 2000; 71:1698S–1702S. PMID: 10837321.
Article28. Fichtner-Feigl S, Fuss IJ, Preiss JC, Strober W, Kitani A. Treatment of murine Th1- and Th2-mediated inflammatory bowel disease with NF-kappa B decoy oligonucleotides. J Clin Invest. 2005; 115:3057–3071. PMID: 16239967.29. Klotz L, Schmidt M, Giese T, Sastre M, Knolle P, Klockgether T, Heneka MT. Proinflammatory stimulation and pioglitazone treatment regulate peroxisome proliferator-activated receptor gamma levels in peripheral blood mononuclear cells from healthy controls and multiple sclerosis patients. J Immunol. 2005; 175:4948–4955. PMID: 16210596.30. Ponchel F, Morgan AW, Bingham SJ, Quinn M, Buch M, Verburg RJ, Henwood J, Douglas SH, Masurel A, Conaghan P, Gesinde M, Taylor J, Markham AF, Emery P, van Laar JM, Isaacs JD. Dysregulated lymphocyte proliferation and differentiation in patients with rheumatoid arthritis. Blood. 2002; 100:4550–4556. PMID: 12393721.
Article31. Vernooy JH, Dentener MA, van Suylen RJ, Buurman WA, Wouters EF. Long-term intratracheal lipopolysaccharide exposure in mice results in chronic lung inflammation and persistent pathology. Am J Respir Cell Mol Biol. 2002; 26:152–159. PMID: 11751215.
Article32. Sakagami T, Vella J, Dixon MF, O'Rourke J, Radcliff F, Sutton P, Shimoyama T, Beagley K, Lee A. The endotoxin of Helicobacter pylori is a modulator of host-dependent gastritis. Infect Immun. 1997; 65:3310–3316. PMID: 9234792.
Article33. Ishiguro Y. Mucosal proinflammatory cytokine production correlates with endoscopic activity of ulcerative colitis. J Gastroenterol. 1999; 34:66–74. PMID: 10204613.
Article34. Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003; 3:521–533. PMID: 12876555.
Article35. Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006; 83:1505S–1519S. PMID: 16841861.
Article36. Kawasaki T, Fujimi S, Lederer JA, Hubbard WJ, Choudhry MA, Schwacha MG, Bland KI, Chaudry IH. Trauma-hemorrhage induces depressed splenic dendritic cell functions in mice. J Immunol. 2006; 177:4514–4520. PMID: 16982888.
Article37. Palombo EA. Traditional medicinal plant extracts and natural products with activity against oral bacteria: potential application in the Prevention and treatment of oral diseases. Evid Based Complement Alternat Med. 2011; 2011:680354. PMID: 19596745.
Article38. Sergent T, Vanderstraeten J, Winand J, Beguin P, Schneider YJ. Phenolic compounds and plant extracts as potential natural anti-obesity substances. Food Chem. 2012; 135:68–73.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anti-Inflammatory and Anti-Superbacterial Activity of Polyphenols Isolated from Black Raspberry
- Antioxidant and Anti-inflammatory Activity of Six Halophytes in Korea
- Anti-nociceptive and Anti-inflammatory Effect of an Ethanol Extract Mixture of Vitis amurensis, Aralia cordata, and Glycyrrhizae radix
- Molecular Targets of Dietary Polyphenols with Anti-inflammatory Properties
- Antioxidant and Anti-inflammatory Activities of Rumex acetosa