Korean J Physiol Pharmacol.
2013 Dec;17(6):511-516. 10.4196/kjpp.2013.17.6.511.
The TREK2 Channel Is Involved in the Proliferation of 253J Cell, a Human Bladder Carcinoma Cell
- Affiliations
-
- 1Division of Integrative Biosciences and Biotechnology, Pohang University of Science and Technology, Pohang 790-784, Korea.
- 2Department of Biochemistry, Dongeui University College of Oriental Medicine, Busan 614-714, Korea.
- 3Department of Physiology, College of Medicine, Chungbuk National University, Cheongju 361-763, Korea. yangmik@chungbuk.ac.kr
- 4Department of Biomedical Engineering, College of Medicine, Chungbuk National University, Cheongju 361-763, Korea.
- 5Department of Urology, College of Medicine, Chungbuk National University, Cheongju 361-763, Korea.
- 6Personalized Tumor Engineering Research Center, College of Medicine, Chungbuk National University, Cheongju 361-763, Korea.
- KMID: 2285478
- DOI: http://doi.org/10.4196/kjpp.2013.17.6.511
Abstract
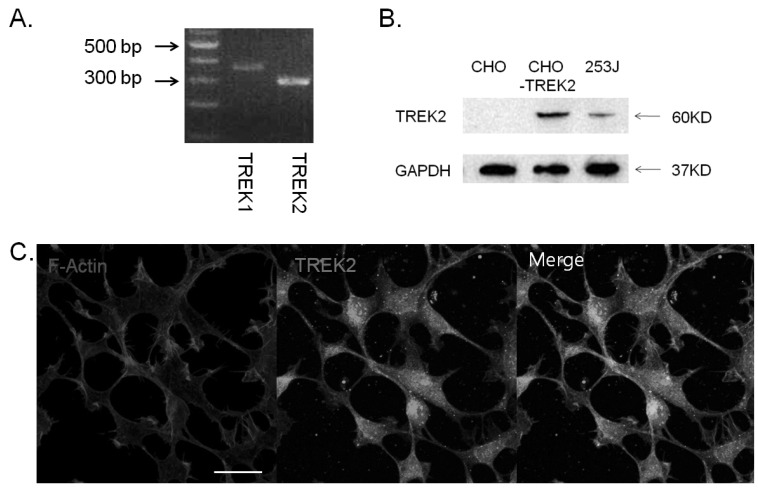
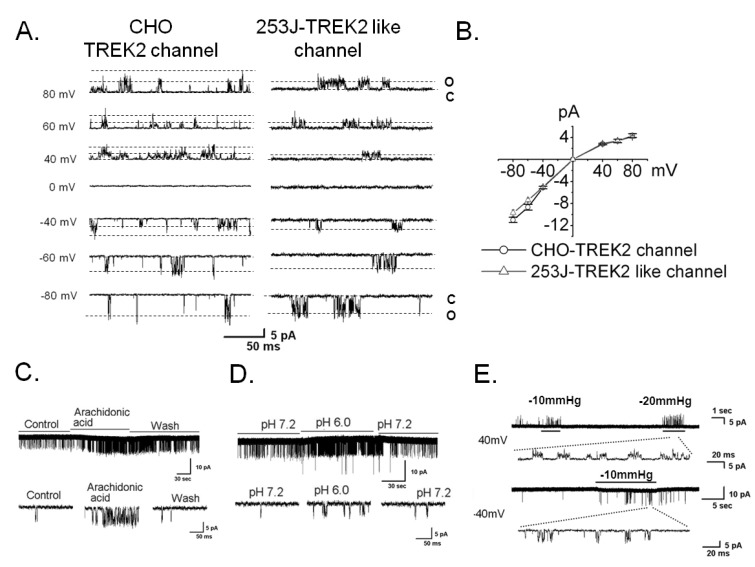
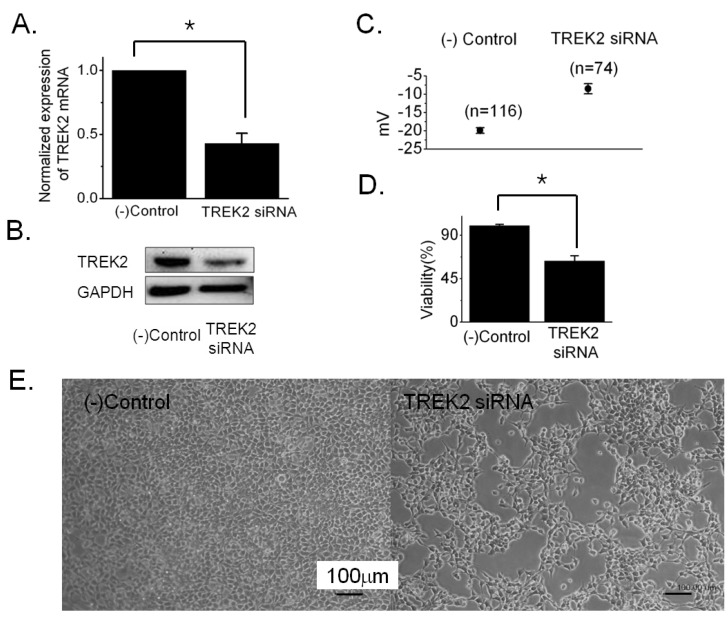
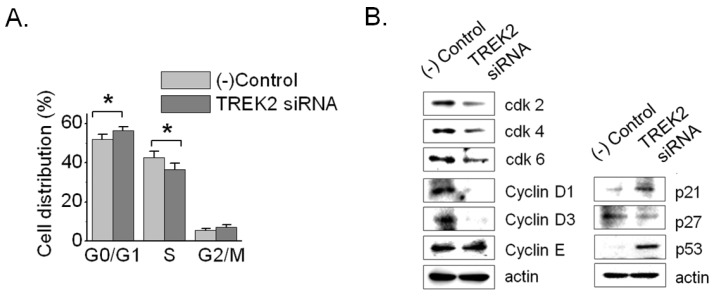
- Bladder cancer is the seventh most common cancer in men that smoke, and the incidence of disease increases with age. The mechanism of occurrence has not yet been established. Potassium channels have been linked with cell proliferation. Some two-pore domain K+ channels (K2P), such as TASK3 and TREK1, have recently been shown to be overexpressed in cancer cells. Here we focused on the relationship between cell growth and the mechanosensitive K2P channel, TREK2, in the human bladder cancer cell line, 253J. We confirmed that TREK2 was expressed in bladder cancer cell lines by Western blot and quantitative real-time PCR. Using the patch-clamp technique, the mechanosensitive TREK2 channel was recorded in the presence of symmetrical 150 mM KCl solutions. In 253J cells, the TREK2 channel was activated by polyunsaturated fatty acids, intracellular acidosis at -60 mV and mechanical stretch at -40 mV or 40 mV. Furthermore, small interfering RNA (siRNA)-mediated TREK2 knockdown resulted in a slight depolarization from -19.9 mV+/-0.8 (n=116) to -8.5 mV+/-1.4 (n=74) and decreased proliferation of 253J cells, compared to negative control siRNA. 253J cells treated with TREK2 siRNA showed a significant increase in the expression of cell cycle boundary proteins p21 and p53 and also a remarkable decrease in protein expression of cyclins D1 and D3. Taken together, the TREK2 channel is present in bladder cancer cell lines and may, at least in part, contribute to cell cycle-dependent growth.
Keyword
MeSH Terms
-
Acidosis
Blotting, Western
Cell Cycle
Cell Line
Cell Proliferation
Cyclins
Fatty Acids, Unsaturated
Humans*
Incidence
Male
Patch-Clamp Techniques
Potassium Channels
Real-Time Polymerase Chain Reaction
RNA, Small Interfering
Smoke
Urinary Bladder Neoplasms
Urinary Bladder*
Cyclins
Fatty Acids, Unsaturated
Potassium Channels
RNA, Small Interfering
Smoke
Figure
Cited by 1 articles
-
Activation of K+ channel by 1-EBIO rescues the head and neck squamous cell carcinoma cells from Ca2+ ionophore-induced cell death
Ming Zhe Yin, Seok-Woo Park, Tae Wook Kang, Kyung Soo Kim, Hae Young Yoo, Junho Lee, J. Hun Hah, Myung Hun Sung, Sung Joon Kim
Korean J Physiol Pharmacol. 2016;20(1):25-33. doi: 10.4196/kjpp.2016.20.1.25.
Reference
-
1. Becchetti A. Ion channels and transporters in cancer 1 Ion channels and cell proliferation in cancer. Am J Physiol Cell Physiol. 2011; 301:C255–C265. PMID: 21430288.
Article3. Ouadid-Ahidouch H, Ahidouch A. K+ channel expression in human breast cancer cells: involvement in cell cycle regulation and carcinogenesis. J Membr Biol. 2008; 221:1–6. PMID: 18060344.4. Li M, Xiong ZG. Ion channels as targets for cancer therapy. Int J Physiol Pathophysiol Pharmacol. 2011; 3:156–166. PMID: 21760973.5. Es-Salah-Lamoureux Z, Steele DF, Fedida D. Research into the therapeutic roles of two-pore-domain potassium channels. Trends Pharmacol Sci. 2010; 31:587–595. PMID: 20951446.
Article6. Kim KA, Kim Y. The effect of flavonoids on the TREK-1 channel. J Korea Acad-Ind Coop Soc. 2011; 12:2660–2667.
Article7. Pei L, Wiser O, Slavin A, Mu D, Powers S, Jan LY, Hoey T. Oncogenic potential of TASK3 (Kcnk9) depends on K+ channel function. Proc Natl Acad Sci U S A. 2003; 100:7803–7807. PMID: 12782791.8. Voloshyna I, Besana A, Castillo M, Matos T, Weinstein IB, Mansukhani M, Robinson RB, Cordon-Cardo C, Feinmark SJ. TREK-1 is a novel molecular target in prostate cancer. Cancer Res. 2008; 68:1197–1203. PMID: 18281496.
Article9. Innamaa A, Jackson L, Asher V, van Schalkwyk G, Warren A, Keightley A, Hay D, Bali A, Sowter H, Khan R. Expression and effects of modulation of the K2P potassium channels TREK-1 (KCNK2) and TREK-2 (KCNK10) in the normal human ovary and epithelial ovarian cancer. Clin Transl Oncol. 2013; 15:910–918. PMID: 23479219.
Article10. Kakehi Y, Hirao Y, Kim WJ, Ozono S, Masumori N, Miyanaga N, Nasu Y, Yokomizo A. Bladder Cancer Working Group report. Jpn J Clin Oncol. 2010; 40(Suppl 1):i57–i64. PMID: 20870921.
Article11. Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009; 27:289–293. PMID: 19219610.
Article12. Kim Y, Kim WJ, Cha EJ. Quercetin-induced growth inhibition in human bladder cancer cells is associated with an increase in Ca2+-activated K+ channels. Korean J Physiol Pharmacol. 2011; 15:279–283. PMID: 22128260.13. Yamada T, Ueda T, Shibata Y, Ikegami Y, Saito M, Ishida Y, Ugawa S, Kohri K, Shimada S. TRPV2 activation induces apoptotic cell death in human T24 bladder cancer cells: a potential therapeutic target for bladder cancer. Urology. 2010; 76:509.e1. 509.e7. PMID: 20546877.
Article14. Li Q, Wang XH, Yang ZH, Wang HP, Yang ZW, Li SW, Zheng XM. [Induction of cell cycle arrest in bladder cancer RT4 cells by capsaicin]. Zhonghua Yi Xue Za Zhi. 2010; 90:1230–1233. PMID: 20646592.15. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981; 391:85–100. PMID: 6270629.
Article16. Bang H, Kim Y, Kim D. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. J Biol Chem. 2000; 275:17412–17419. PMID: 10747911.17. Rouzaire-Dubois B, Dubois JM. K+ channel block-induced mammalian neuroblastoma cell swelling: a possible mechanism to influence proliferation. J Physiol. 1998; 510(Pt 1):93–102. PMID: 9625869.18. Wonderlin WF, Strobl JS. Potassium channels, proliferation and G1 progression. J Membr Biol. 1996; 154:91–107. PMID: 8929284.
Article19. Ghiani CA, Yuan X, Eisen AM, Knutson PL, DePinho RA, McBain CJ, Gallo V. Voltage-activated K+ channels and membrane depolarization regulate accumulation of the cyclin-dependent kinase inhibitors p27(Kip1) and p21(CIP1) in glial progenitor cells. J Neurosci. 1999; 19:5380–5392. PMID: 10377348.20. Woodfork KA, Wonderlin WF, Peterson VA, Strobl JS. Inhibition of ATP-sensitive potassium channels causes reversible cell-cycle arrest of human breast cancer cells in tissue culture. J Cell Physiol. 1995; 162:163–171. PMID: 7822427.
Article21. Smith GA, Tsui HW, Newell EW, Jiang X, Zhu XP, Tsui FW, Schlichter LC. Functional up-regulation of HERG K+ channels in neoplastic hematopoietic cells. J Biol Chem. 2002; 277:18528–18534. PMID: 11893742.22. Wonderlin WF, Woodfork KA, Strobl JS. Changes in membrane potential during the progression of MCF-7 human mammary tumor cells through the cell cycle. J Cell Physiol. 1995; 165:177–185. PMID: 7559799.
Article23. Ouadid-Ahidouch H, Le Bourhis X, Roudbaraki M, Toillon RA, Delcourt P, Prevarskaya N. Changes in the K+ current-density of MCF-7 cells during progression through the cell cycle: possible involvement of a h-ether.a-gogo K+ channel. Receptors Channels. 2001; 7:345–356. PMID: 11697078.24. Ouadid-Ahidouch H, Roudbaraki M, Delcourt P, Ahidouch A, Joury N, Prevarskaya N. Functional and molecular identification of intermediate-conductance Ca2+-activated K+ channels in breast cancer cells: association with cell cycle progression. Am J Physiol Cell Physiol. 2004; 287:C125–C134. PMID: 14985237.25. Borowiec AS, Hague F, Gouilleux-Gruart V, Lassoued K, Ouadid-Ahidouch H. Regulation of IGF-1-dependent cyclin D1 and E expression by hEag1 channels in MCF-7 cells: the critical role of hEag1 channels in G1 phase progression. Biochim Biophys Acta. 2011; 1813:723–730. PMID: 21315112.
Article26. Vermeulen K, Van Bockstaele DR, Berneman ZN. The cell cycle: a review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003; 36:131–149. PMID: 12814430.
Article27. Park KS, Kim Y. Functional expression of mechanosensitive two-pore domain potassium channel in human bladder carcinoma cells. J Biomed Res. 2013; 14:71–76.
Article28. Kang D, Kim D. TREK-2 (K2P101) and TRESK (K2P181) are major background K+ channels in dorsal root ganglion neurons. Am J Physiol Cell Physiol. 2006; 291:C138–C146. PMID: 16495368.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Functional expression of mechanosensitive two-pore domain potassium channel in human bladder carcinoma cells
- Altered Expression of Genes in a Human Urinary Bladder Transitional Cell Carcinoma Cell Line of Low-metastatic Potential and Its Subclone of High-metastatic Potential
- Growth Inhibition After Exposure to Transforming Growth Factor-beta1 in Human Bladder Cancer Cell Lines
- The Inhibition of TREK2 Channel by an Oxidizing Agent, 5,5'-dithio- bis (2-nitrobenzoic acid), via Interaction with the C-terminus Distal to the 353rd Amino Acid
- Papillary Transitional Cell Carcinoma of the Bladder: Report of a Case