Korean J Urol.
2014 Jul;55(7):487-492. 10.4111/kju.2014.55.7.487.
Growth Inhibition After Exposure to Transforming Growth Factor-beta1 in Human Bladder Cancer Cell Lines
- Affiliations
-
- 1Department of Urology, Soonchunhyang University Cheonan Hospital, Cheonan, Korea.
- 2Department of Urology, Seoul National University College of Medicine, Seoul, Korea. selee@snubh.org
- 3Department of Biochemistry, Soonchunhyang University College of Medicine, Cheonan, Korea.
- 4Department of Urology, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 1794579
- DOI: http://doi.org/10.4111/kju.2014.55.7.487
Abstract
- PURPOSE
Transforming growth factor-beta1 (TGF-beta1) plays a dual role in apoptosis and in proapoptotic responses in the support of survival in a variety of cells. The aim of this study was to determine the function of TGF-beta1 in bladder cancer cells.
MATERIALS AND METHODS
The role of TGF-beta1 in bladder cancer cells was examined by observing cell viability by using the tetrazolium dye (MTT) assay after treating the bladder cancer cell lines 253J, 5637, T24, J82, HT1197, and HT1376 with TGF-beta1. Among these cell lines, the 253J and T24 cell lines were coincubated with TGF-beta1 and the pan anti-TGF-beta antibody. Fluorescence-activated cell sorter (FACS) analysis was performed to determine the mechanism involved after TGF-beta1 treatment in 253J cells.
RESULTS
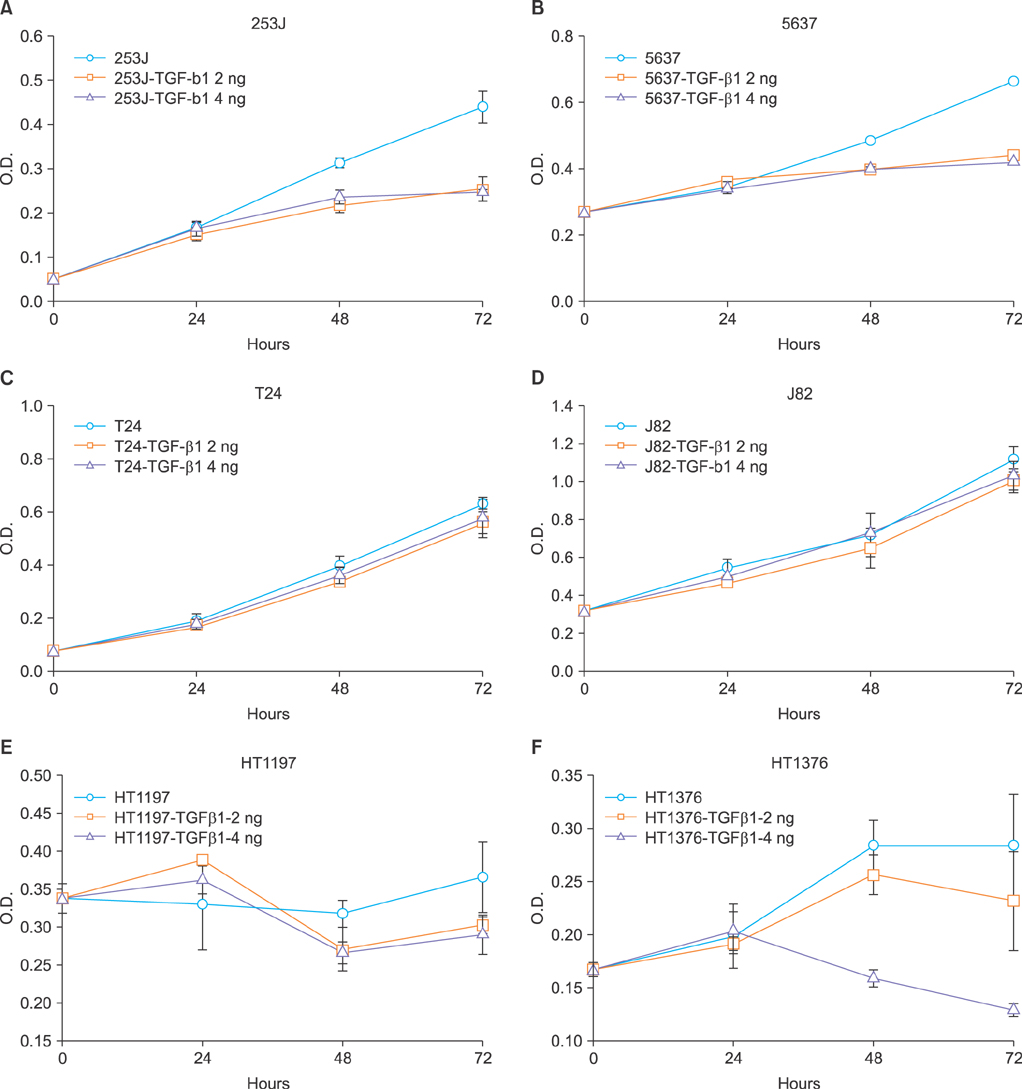
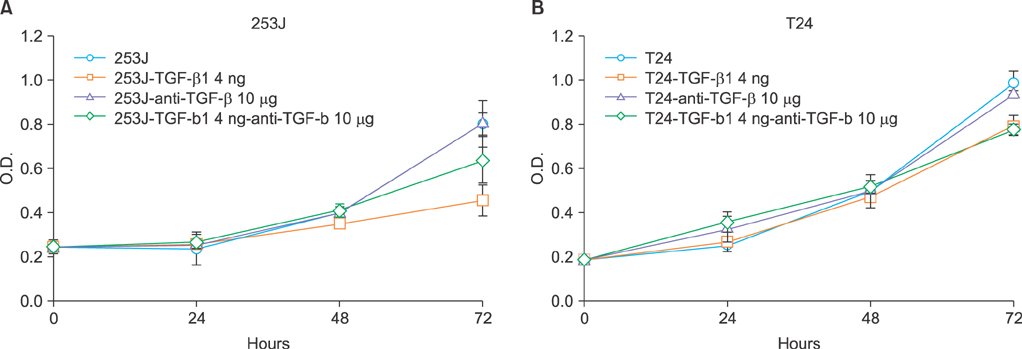
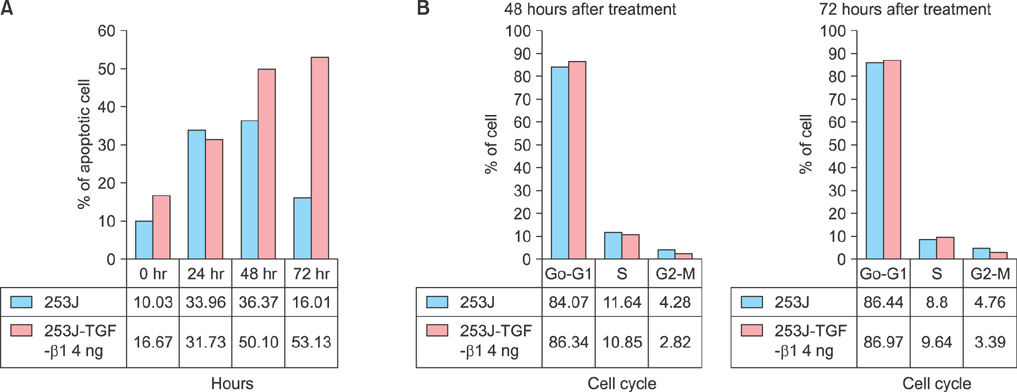
All six cell lines showed inhibited cellular growth after TGF-beta1 treatment. Although the T24 and J82 cell lines also showed inhibited cellular growth, the growth inhibition was less than that observed in the other 4 cell lines. The addition of pan anti-TGF-beta antibodies to the culture media restored the growth properties that had been inhibited by TGF-beta1. FACS analysis was performed in the 253J cells and the 253J cells with TGF-beta1. There were no significant differences in the cell cycle between the two treatments. However, there were more apoptotic cells in the TGF-beta1-treated 253J cells.
CONCLUSIONS
TGF-beta1 did not stimulate cellular proliferation but was a growth inhibitory factor in bladder cancer cells. However, the pattern of its effects depended on the cell line. TGF-beta1 achieved growth inhibition by enhancing the level of apoptosis.
MeSH Terms
-
Antineoplastic Agents/administration & dosage/*pharmacology
Apoptosis/drug effects
Cell Line, Tumor/drug effects/pathology
Cell Proliferation/drug effects
Cell Separation/methods
Dose-Response Relationship, Drug
Drug Screening Assays, Antitumor/methods
Flow Cytometry/methods
Humans
Transforming Growth Factor beta1/administration & dosage/*pharmacology
Urinary Bladder Neoplasms/*pathology
Antineoplastic Agents
Transforming Growth Factor beta1
Figure
Reference
-
1. Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor beta in human disease. N Engl J Med. 2000; 342:1350–1358.2. Govinden R, Bhoola KD. Genealogy, expression, and cellular function of transforming growth factor-beta. Pharmacol Ther. 2003; 98:257–265.3. Sanchez-Capelo A. Dual role for TGF-beta1 in apoptosis. Cytokine Growth Factor Rev. 2005; 16:15–34.4. Roberts AB, Sporn MB. Transforming growth factors. Cancer Surv. 1985; 4:683–705.5. Roberts AB, Wakefield LM. The two faces of transforming growth factor beta in carcinogenesis. Proc Natl Acad Sci U S A. 2003; 100:8621–8623.6. Pasche B. Role of transforming growth factor beta in cancer. J Cell Physiol. 2001; 186:153–168.7. Reiss M. TGF-beta and cancer. Microbes Infect. 1999; 1:1327–1347.8. Kirkali Z, Chan T, Manoharan M, Algaba F, Busch C, Cheng L, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. In : Soloway M, Carmack A, Khoury S, editors. Bladder tumors. Paris: Health Publication Ltd;2005. p. 13–64.9. Ministry of Health and Welfare, Korea Central Cancer Registry, National Cancer Center. Annual report of cancer statistics in Korea in 2010. Goyang: Ministry of Health and Welfare, Korea Central Cancer Registry, National Cancer Center;2011.10. Roberts AB. Molecular and cell biology of TGF-beta. Miner Electrolyte Metab. 1998; 24:111–119.11. Eder IE, Stenzl A, Hobisch A, Cronauer MV, Bartsch G, Klocker H. Expression of transforming growth factors beta-1, beta 2 and beta 3 in human bladder carcinomas. Br J Cancer. 1997; 75:1753–1760.12. Park BJ, Park JI, Byun DS, Park JH, Chi SG. Mitogenic conversion of transforming growth factor-beta1 effect by oncogenic Ha-Ras-induced activation of the mitogen-activated protein kinase signaling pathway in human prostate cancer. Cancer Res. 2000; 60:3031–3038.13. Champelovier P, El Atifi M, Mantel F, Rostaing B, Simon A, Berger F, et al. In vitro tumoral progression of human bladder carcinoma: role for TGFbeta. Eur Urol. 2005; 48:846–851.14. Shin KY, Kim SJ, Choi WH, Choi DY, Park HY, Lee TY, et al. A study in the effects of epidermal growth factor and transforming growth factors-alpha on the growth of human transitional cell carcinoma cell lines. Korean J Urol. 1998; 39:45–50.15. Iles RK. Ectopic hCGbeta expression by epithelial cancer: malignant behaviour, metastasis and inhibition of tumor cell apoptosis. Mol Cell Endocrinol. 2007; 260-262:264–270.16. Kim JH, Shariat SF, Kim IY, Menesses-Diaz A, Tokunaga H, Wheeler TM, et al. Predictive value of expression of transforming growth factor-beta(1) and its receptors in transitional cell carcinoma of the urinary bladder. Cancer. 2001; 92:1475–1483.17. Sanchez-Carbayo M, Socci ND, Charytonowicz E, Lu M, Prystowsky M, Childs G, et al. Molecular profiling of bladder cancer using cDNA microarrays: defining histogenesis and biological phenotypes. Cancer Res. 2002; 62:6973–6980.18. Elliott AY, Cleveland P, Cervenka J, Castro AE, Stein N, Hakala TR, et al. Characterization of a cell line from human transitional cell cancer of the urinary tract. J Natl Cancer Inst. 1974; 53:1341–1349.19. Ko TC, Sheng HM, Reisman D, Thompson EA, Beauchamp RD. Transforming growth factor-beta 1 inhibits cyclin D1 expression in intestinal epithelial cells. Oncogene. 1995; 10:177–184.20. Geng Y, Weinberg RA. Transforming growth factor beta effects on expression of G1 cyclins and cyclin-dependent protein kinases. Proc Natl Acad Sci U S A. 1993; 90:10315–10319.21. Ravitz MJ, Wenner CE. Cyclin-dependent kinase regulation during G1 phase and cell cycle regulation by TGF-beta. Adv Cancer Res. 1997; 71:165–207.22. Carneiro C, Alvarez CV, Zalvide J, Vidal A, Dominguez F. TGF-beta1 actions on FRTL-5 cells provide a model for the physiological regulation of thyroid growth. Oncogene. 1998; 16:1455–1465.23. Al-Azayzih A, Gao F, Goc A, Somanath PR. TGFβ1 induces apoptosis in invasive prostate cancer and bladder cancer cells via Akt-independent, p38 MAPK and JNK/SAPK-mediated activation of caspases. Biochem Biophys Res Commun. 2012; 427:165–170.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inhibition of Cell Growth and Suppression of c-myc Gene Expression by Transforming Growth Factor-beta1 in Cervical Carcinoma Cell Lines
- The Expression of TGF-beta1 and TGF-beta Receptor I in Human Lung Cancer
- The Transforming Growth Factor-beta1 Expression in Normal Laryngeal Mucosa, Laryngeal Dysplasia and Laryngeal Carcinoma
- Effects of Transforming Growth Factor-beta1 and Its Receptor on the Development, Recurrence and Progression of Human Bladder Cancer
- Effect of Transforming Growth Factor-beta1 on Expressions of Epidermal Growth Factor and Transforming Growth Factor-alpha in DU145 Androgen-Independent Prostate Cancer Cells