Korean J Physiol Pharmacol.
2009 Dec;13(6):491-496. 10.4196/kjpp.2009.13.6.491.
p38 MAPK Participates in Muscle-Specific RING Finger 1-Mediated Atrophy in Cast-Immobilized Rat Gastrocnemius Muscle
- Affiliations
-
- 1Department of Physical Therapy, College of Public Health & Welfare, Yongin University, Yongin 449-714, Korea.
- 2Department of Physiology, Institute of Functional Genomics, School of Medicine, Konkuk University, Choongju 380-701, Korea. bkkim2@kku.ac.kr
- 3Department of Herbal Medicine, College of Natural Sciences, Hoseo University, Asan 336-795, Korea.
- 4Bio Food and Drug Research Center, Konkuk University, Choongju 380-701, Korea.
- KMID: 2285386
- DOI: http://doi.org/10.4196/kjpp.2009.13.6.491
Abstract
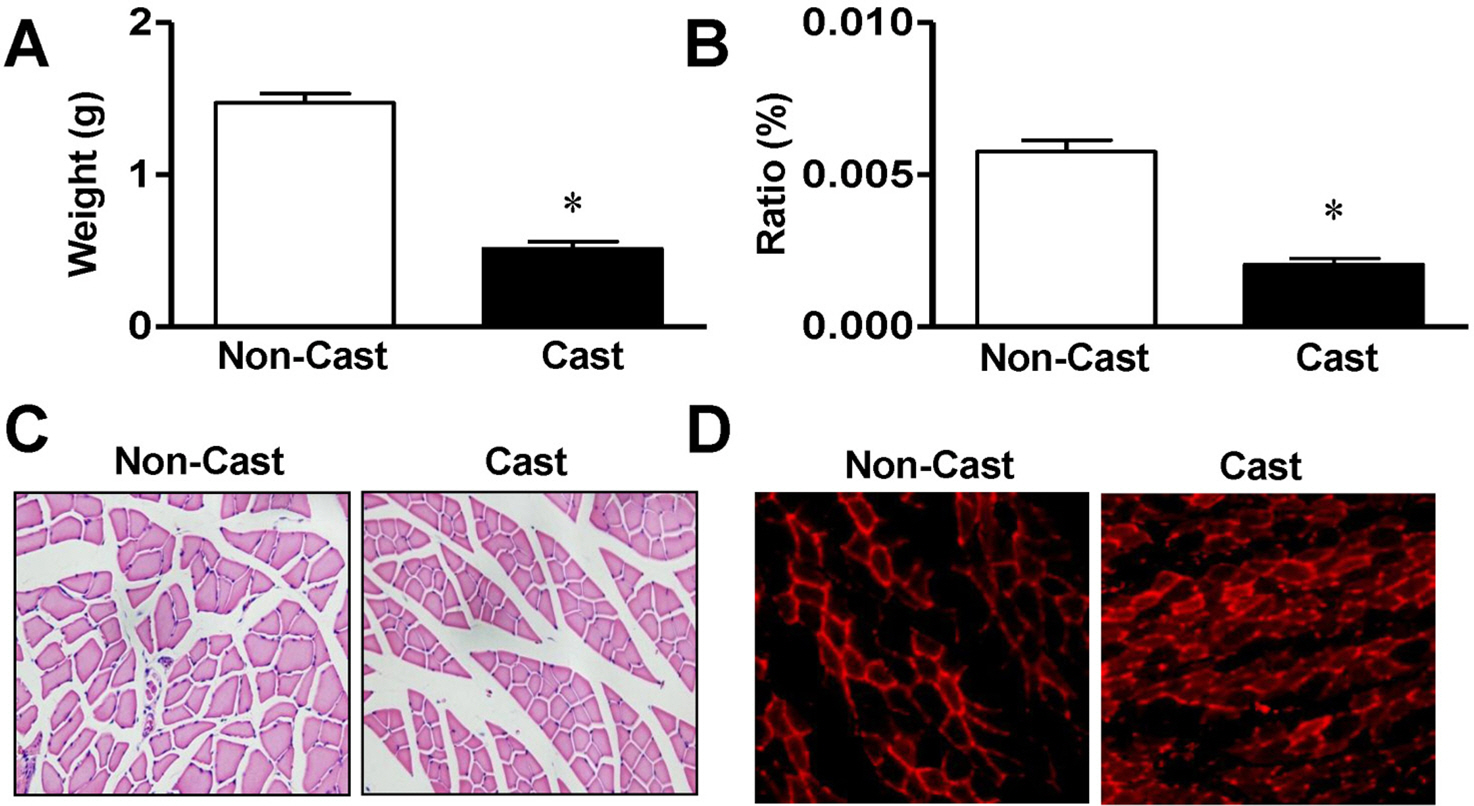
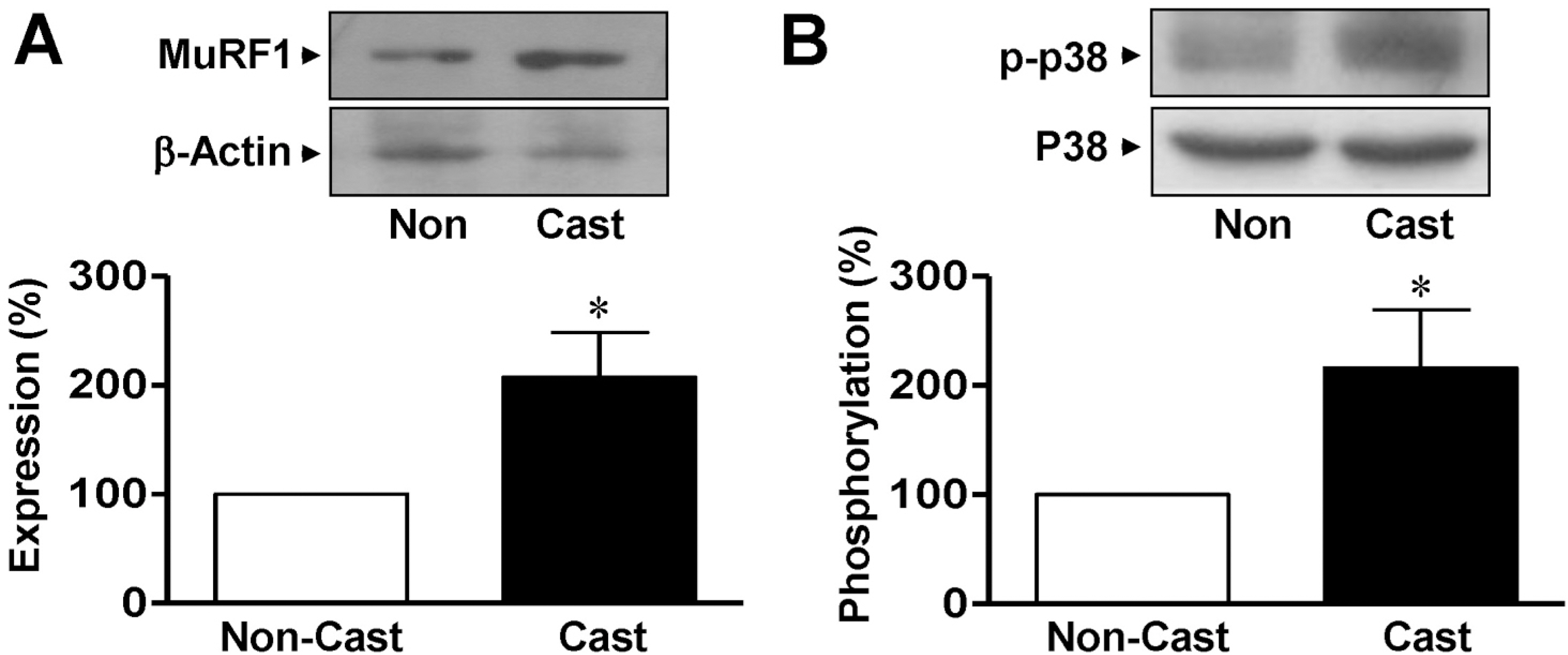
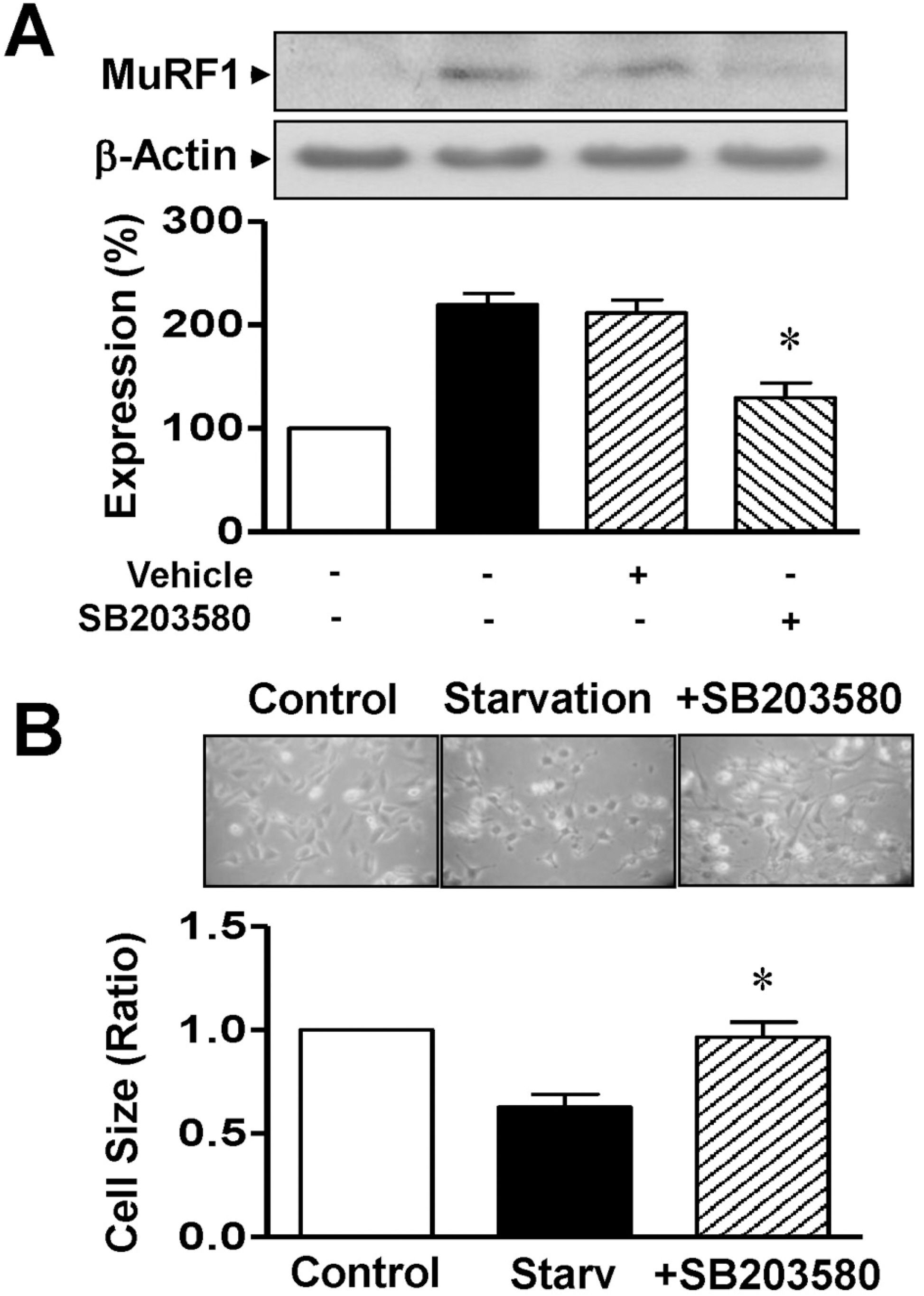
- Skeletal muscle atrophy is a common phenomenon during the prolonged muscle disuse caused by cast immobilization, extended aging states, bed rest, space flight, or other factors. However, the cellular mechanisms of the atrophic process are poorly understood. In this study, we investigated the involvement of mitogen-activated protein kinase (MAPK) in the expression of muscle-specific RING finger 1 (MuRF1) during atrophy of the rat gastrocnemius muscle. Histological analysis revealed that cast immobilization induced the atrophy of the gastrocnemius muscle, with diminution of muscle weight and cross-sectional area after 14 days. Cast immobilization significantly elevated the expression of MuRF1 and the phosphorylation of p38 MAPK. The starvation of L6 rat skeletal myoblasts under serum-free conditions induced the phosphorylation of p38 MAPK and the characteristics typical of cast-immobilized gastrocnemius muscle. The expression of MuRF1 was also elevated in serum-starved L6 myoblasts, but was significantly attenuated by SB203580, an inhibitor of p38 MAPK. Changes in the sizes of L6 myoblasts in response to starvation were also reversed by their transfection with MuRF1 small interfering RNA or treatment with SB203580. From these results, we suggest that the expression of MuRF1 in cast-immobilized atrophy is regulated by p38 MAPK in rat gastrocnemius muscles.
MeSH Terms
-
Aging
Animals
Atrophy
Bed Rest
Fingers
Imidazoles
Immobilization
Muscle, Skeletal
Muscles
Myoblasts
Myoblasts, Skeletal
p38 Mitogen-Activated Protein Kinases
Phosphorylation
Protein Kinases
Pyridines
Rats
RNA, Small Interfering
Space Flight
Starvation
Transfection
Imidazoles
Protein Kinases
Pyridines
RNA, Small Interfering
p38 Mitogen-Activated Protein Kinases
Figure
Reference
-
References
Benveniste O., Jacobson L., Farrugia ME., Clover L., Vincent A. MuSK antibody positive myasthenia gravis plasma modifies MuRF-1 expression in C2C12 cultures and mouse muscle in vivo. J Neuroimmunol. 170:41–48. 2005.
ArticleBodine SC., Latres E., Baumhueter S., Lai VK., Nunez L., Clarke BA., Poueymirou WT., Panaro FJ., Na E., Dharmarajan K., Pan ZQ., Valenzuela DM., DeChiara TM., Stitt TN., Yancopoulos GD., Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 294:1704–1708. 2001.
ArticleBooth FW., Kelso JR. Production of rat muscle atrophy by cast fixation. Appl Physiol. 34:404–406. 1973.
ArticleBooth FW., Thomason DB. Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiol Rev. 71:541–585. 1991.
ArticleCai D., Frantz JD., Jr Tawa NE., Melendez PA., Oh BC., Lidov HG., Hasselgren PO., Frontera WR., Lee J., Glass DJ., Shoelson SE. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell. 119:285–298. 2004.Centner T., Yano J., Kimura E., McElhinny AS., Pelin K., Witt CC., Bang ML., Trombitas K., Granzier H., Gregorio CC., Sorimachi H., Labeit S. Identification of muscle specific ring finger proteins as potential regulators of the titin kinase domain. J Mol Biol. 306:717–726. 2001.
ArticleChang L., Karin K. Mammalian MAP kinase signalling cascades. Nature. 410:37–40. 2001.
ArticleFreemont PS. RING for destruction? Curr Biol. 10:R84–R87. 2000.Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 37:1974–1984. 2005.
ArticleGomes MD., Lecker SH., Jagoe RT., Navon A., Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci USA. 98:14440–14445. 2001.
ArticleHilder TL., Tou JC., Grindeland RE., Wade CE., Graves LM. Phosphorylation of insulin receptor substrate-1 serine 307 correlates with JNK activity in atrophic skeletal muscle. FEBS Lett. 553:63–67. 2003.
ArticleJagoe RT., Goldberg AL. What do we really know about the ubiquitin-proteasome pathway in muscle atrophy. Curr Opin Clin Nutr Metab Care. 4:183–190. 2001.
ArticleKedar V., McDonough H., Arya R., Li HH., Rockman HA., Patterson C. Muscle-specific RING finger 1 is a bona fide ubiquitin ligase that degrades cardiac troponin I. Proc Natl Acad Sci USA. 101:18135–18140. 2004.
ArticleKyriakis JM., Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 81:807–869. 2001.Lecker SH., Jagoe RT., Gilbert A., Gomes M., Baracos V., Bailey J., Price SR., Mitch WE., Goldberg AL. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 18:39–51. 2004.
ArticleLee HM., Jeon BH., Won KJ., Lee CK., Park TK., Choi WS., Bae YM., Kim HS., Lee SK., Park SH., Irani K., Kim B. Gene transfer of redox factor-1 inhibits neointimal formation: involvement of platelet-derived growth factor-beta receptor signaling via the inhibition of the reactive oxygen species-mediated Syk pathway. Cir Res. 104:219–227. 2009.Lee HM., Lee CK., Lee SH., Roh HY., Bae YM., Lee KY., Lim J., Park PJ., Park TK., Lee YL., Won KJ., Kim B. p38 mitogen-activated protein kinase contributes to angiotensin II-stimulated migration of rat aortic smooth muscle cells. J Pharmacol Sci. 105:74–81. 2007.
ArticleLi YP., Chen Y., John J., Moylan J., Jin B., Mann DL., Reid MB. TNF-α acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 19:362–370. 2005.Machida S., Booth FW. Changes in signalling molecule levels in 10-day hindlimb immobilized rat muscles. Acta Physiol Scand. 183:171–179. 2005.
ArticleMcElhinny AS., Kakinuma K., Sorimachi H., Labeit S., Gregorio CC. Muscle-specific RING finger-1 interacts with titin to regulate sarcomeric M-line and thick filament structure and may have nuclear functions via its interaction with glucocorticoid modulatory element binding protein-1. J Cell Biol. 157:125–136. 2002.
ArticleMcKinnell IW., Rudnicki MA. Molecular mechanisms of muscle atrophy. Cell. 119:907–910. 2004.
ArticleMedina R., Wing SS., Goldberg AL. Increase in levels of polyubiquitin and proteasome mRNA in skeletal muscle during starvation and denervation atrophy. Biochem J. 307:631–637. 1995.
ArticleNader GA. Molecular determinants of skeletal muscle mass: getting the “AKT” together. Int J Biochem Cell Biol. 37:1985–1996. 2005.
ArticleSamarel AM., Parmacek MS., Magid NM., Decker RS., Lesch M. Protein synthesis and degradation during starvation-induced cardiac atrophy in rabbits. Cir Res. 60:933–941. 1987.
ArticleSandri M., Sandri C., Gilbert A., Skurk C., Calabria E., Picard A., Walsh K., Schiaffino S., Lecker SH., Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 117:399–412. 2004.
ArticleSchulze PC., Fang J., Kassik KA., Gannon J., Cupesi M., MacGillivray C., Lee RT., Rosenthal N. Transgenic overexpression of locally acting insulin-like growth factor-1 inhibits ubiquitin-mediated muscle atrophy in chronic left-ventricular dysfunction. Cir Res. 97:418–426. 2005.
ArticleShi H., Scheffler JM., Zeng C., Pleitner JM., Hannon KM., Grant AL., Gerrard DE. Mitogen-activated protein kinase signaling is necessary for the maintenance of skeletal muscle mass. Am J Physiol Cell Physiol. 296:C1040–C1048. 2009.
ArticleSkurk C., Izumiya Y., Maatz H., Razeghi P., Shiojima I., Sandri M., Sato K., Zeng L., Schiekofer S., Pimentel D., Lecker S., Taegtmeyer H., Goldberg AL., Walsh K. The FOXO3a transcription factor regulates cardiac myocyte size downstream of AKT signaling. J Biol Chem. 280:20814–20823. 2005.
ArticleSugita H., Kaneki M., Sugita M., Yasukawa T., Yasuhara S., Martyn JA. Burn injury impairs insulin-stimulated Akt/PKB activation in skeletal muscle. Am J Physiol Endocrinol Metab. 288:E585–E591. 2005.
ArticleTesseraud S., Métayer-Coustard S., Boussaid S., Crochet S., Audouin E., Derouet M., Seiliez I. Insulin and amino acid availability regulate atrogin-1 in avian QT6 cells. Biochem Biophys Res Commun. 357:181–186. 2007.
ArticleThomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr. 26:389–399. 2007.
ArticleTobimatsu K., Noguchi T., Hosooka T., Sakai M., Inagaki K., Matsuki Y., Hiramatsu R., Kasuga M. Overexpression of the transcriptional coregulator Cited2 protects against glucocorticoid-induced atrophy of C2C12 myotubes. Biochem Biophys Res Commun. 378:399–403. 2009.
ArticleWray CJ., Mammen JM., Hershko DD., Hasselgren PO. Sepsis upregulates the gene expression of multiple ubiquitin ligases in skeletal muscle. Int J Biochem Cell Biol. 35:698–705. 2003.
ArticleYamamoto Y., Hoshino Y., Ito T., Nariai T., Mohri T., Obana M., Hayata N., Uozumi Y., Maeda M., Fujio Y., Azuma J. Atrogin-1 ubiquitin ligase is upregulated by doxorubicin via p38-MAP kinase in cardiac myocytes. Cardiovasc Res. 79:89–96. 2008.
ArticleZhou LZ., Johnson AP., Rando TA. NFκB and AP-1 mediate trans-criptional responses to oxidative stress in skeletal muscle cells. Free Radic Biol Med. 31:1405–1416. 2001.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Aerobic Exercise Ameliorates Muscle Atrophy Induced by Methylglyoxal via Increasing Gastrocnemius and Extensor Digitorum Longus Muscle Sensitivity
- Experimental Studies on Disuse Atrophy of the Rat Tibialis Anterior Muscle
- Beneficial effects of melatonin on stroke-induced muscle atrophy in focal cerebral ischemic rats
- Effect of DHEA on Hindlimb Muscles in a Focal Cerebral Ischemia Model Rat
- Effect of Articular Immobilization - Induced Hindlimb Skeletal Muscle Atrophy on Autophosphorylation of Tyrosine Kinase of the Insulin Receptor in Rats