J Adv Prosthodont.
2014 Dec;6(6):512-520. 10.4047/jap.2014.6.6.512.
Osteoblastic behavior to zirconium coating on Ti-6Al-4V alloy
- Affiliations
-
- 1School of Dentistry, Chonnam National University, Gwangju, Republic of Korea. youngjun@chonnam.ac.kr
- 2Department of Stomatology, Affiliated Hospital of Yanbian University, Yanji, Jilin, China.
- KMID: 2284726
- DOI: http://doi.org/10.4047/jap.2014.6.6.512
Abstract
- PURPOSE
The purpose of this study was to assess the surface characteristics and the biocompatibility of zirconium (Zr) coating on Ti-6Al-4V alloy surface by radio frequency (RF) magnetron sputtering method.
MATERIALS AND METHODS
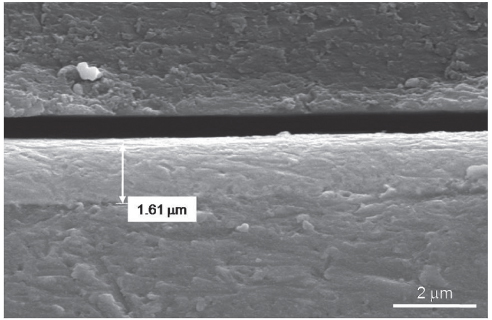
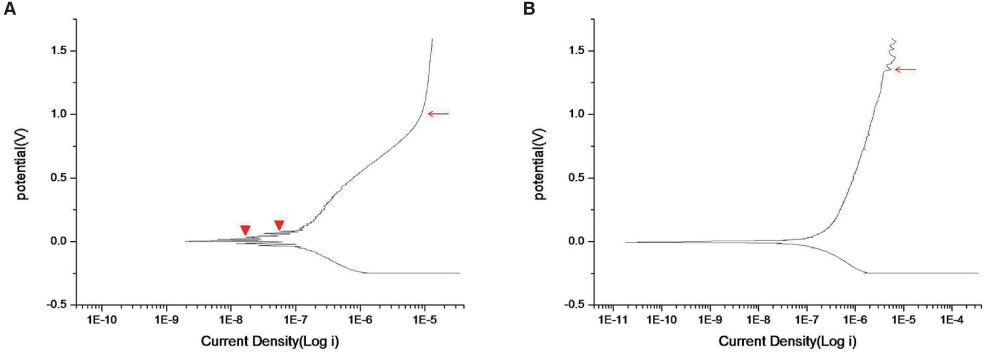
The zirconium films were developed on Ti-6Al-4V discs using RF magnetron sputtering method. Surface profile, surface composition, surface roughness and surface energy were evaluated. Electrochemical test was performed to evaluate the corrosion behavior. Cell proliferation, alkaline phosphatase (ALP) activity and gene expression of mineralized matrix markers were measured.
RESULTS
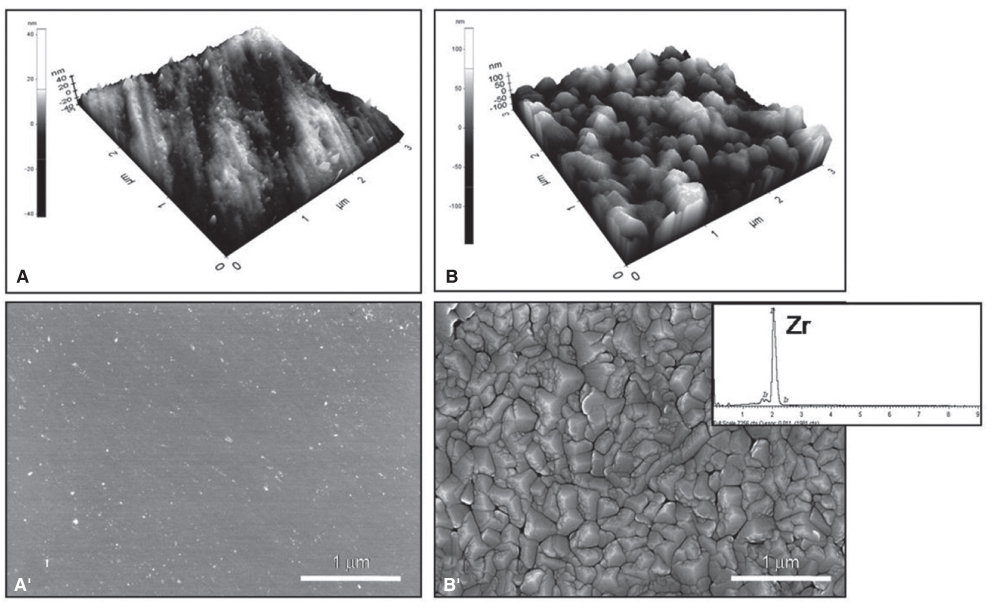
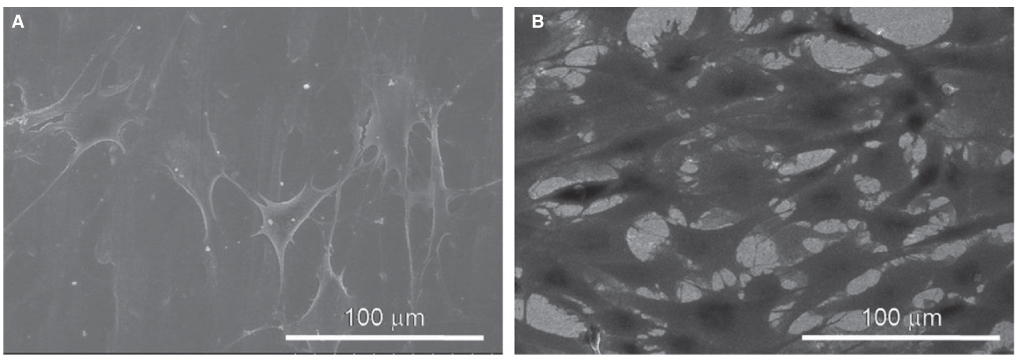
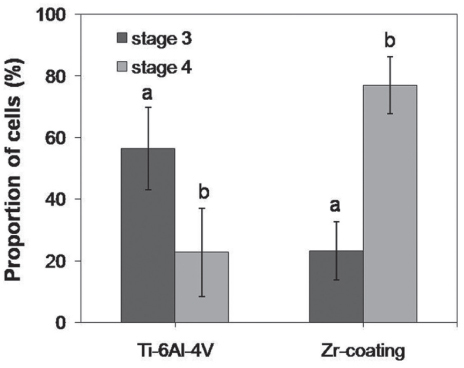
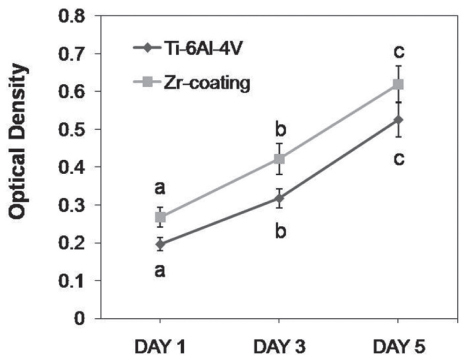
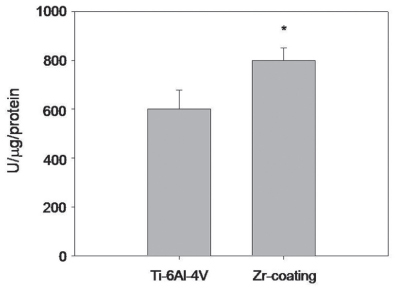
SEM and EDS analysis showed that zirconium deposition was performed successfully on Ti-6Al-4V alloy substrate. Ti-6Al-4V group and Zr-coating group showed no significant difference in surface roughness (P>.05). Surface energy was significantly higher in Zr-coating group than in Ti-6Al-4V group (P<.05). No difference in cell morphology was observed between Ti-6Al-4V group and Zr-coating group. Cell proliferation was higher in Zr-coating group than Ti-6Al-4V group at 1, 3 and 5 days (P<.05). Zr-coating group showed higher ALP activity level than Ti-6Al-4V group (P<.05). The mRNA expressions of bone sialoprotein (BSP) and osteocalcin (OCN) on Zr-coating group increased approximately 1.2-fold and 2.1-fold respectively, compared to that of Ti-6Al-4V group.
CONCLUSION
These results suggest that zirconium coating on Ti-6Al-4V alloy could enhance the early osteoblast responses. This property could make non-toxic metal coatings on Ti-6Al-4V alloy suitable for orthopedic and dental implants.
Keyword
MeSH Terms
-
Alkaline Phosphatase
Alloys*
Biocompatible Materials
Cell Proliferation
Coated Materials, Biocompatible
Corrosion
Dental Implants
Gene Expression
Integrin-Binding Sialoprotein
Orthopedics
Osteoblasts*
Osteocalcin
RNA, Messenger
Surface Properties
Titanium
Zirconium*
Alkaline Phosphatase
Alloys
Biocompatible Materials
Coated Materials, Biocompatible
Dental Implants
Integrin-Binding Sialoprotein
Osteocalcin
RNA, Messenger
Titanium
Zirconium
Figure
Reference
-
1. Park JB, Lakes RS. Biomaterials: an introduction. 2nd ed. New York: Plenum Press;1992. p. 89–92.2. Oshida Y. Bioscience and bioenginerring of titanium materials. 1st ed. New York: Elsevier Press;2007. p. 11–24.3. Khan MA, Williams RL, Williams DF. In-vitro corrosion and wear of titanium alloys in the biological environment. Biomaterials. 1996; 17:2117–2126.4. Khan MA, Williams RL, Williams DF. Conjoint corrosion and wear in titanium alloys. Biomaterials. 1999; 20:765–772.5. Matono Y, Nakagawa M, Matsuya S, Ishikawa K, Terada Y. Corrosion behavior of pure titanium and titanium alloys in various concentrations of Acidulated Phosphate Fluoride (APF) solutions. Dent Mater J. 2006; 25:104–112.6. Okazaki Y, Gotoh E, Manabe T, Kobayashi K. Comparison of metal concentrations in rat tibia tissues with various metallic implants. Biomaterials. 2004; 25:5913–5920.7. Okazaki Y, Gotoh E. Comparison of metal release from various metallic biomaterials in vitro. Biomaterials. 2005; 26:11–21.8. Gomes CC, Moreira LM, Santos VJ, Ramos AS, Lyon JP, Soares CP, Santos FV. Assessment of the genetic risks of a metallic alloy used in medical implants. Genet Mol Biol. 2011; 34:116–121.9. Liu C, Bi Q, Matthews A. Tribological and electrochemical performance of PVD TiN coatings on the femoral head of Ti-6Al-4V artificial hip joints. Surf Coat Technol. 2003; 163-164:597–604.10. Arnould C, Volcke C, Lamarque C, Thiry PA, Delhalle J, Mekhalif Z. Titanium modified with layer-by-layer sol-gel tantalum oxide and an organodiphosphonic acid: a coating for hydroxyapatite growth. J Colloid Interface Sci. 2009; 336:497–503.11. Qian MA, St John DH, Frost MT. Effect of soluble and insoluble zirconium on the grain refinement of magnesium alloys. Mater Sci Forum. 2003; 419-422:593–598.12. Samuel S, Nag S, Nasrazadani S, Ukirde V, El Bouanani M, Mohandas A, Nguyen K, Banerjee R. Corrosion resistance and in vitro response of laser-deposited Ti-Nb-Zr-Ta alloys for orthopedic implant applications. J Biomed Mater Res A. 2010; 94:1251–1256.13. Rosalbino F, Macciò D, Giannoni P, Quarto R, Saccone A. Study of the in vitro corrosion behavior and biocompatibility of Zr-2.5Nb and Zr-1.5Nb-1Ta (at%) crystalline alloys. J Mater Sci Mater Med. 2011; 22:1293–1302.14. Duygulu O, Kaya AA, Oktay G, Sahin FC. Diffusion bonding of magnesium, zirconium and titanium as implant material. Mater Sci Forum. 2007; 546-549:417–420.15. Gómez-Florit M, Xing R, Ramis JM, Taxt-Lamolle S, Haugen HJ, Lyngstadaas SP, Monjo M. Human gingival fibroblasts function is stimulated on machined hydrided titanium zirconium dental implants. J Dent. 2014; 42:30–38.16. Kurella A, Dahotre NB. Laser induced multi-scale textured zirconia coating on Ti-6Al-4V. J Mater Sci Mater Med. 2006; 17:565–572.17. Zhang S, Sun J, Xu Y, Qian S, Wang B, Liu F, Liu X. Adhesion, proliferation and differentiation of osteoblasts on zirconia films prepared by cathodic arc deposition. Biomed Mater Eng. 2013; 23:373–385.18. Sollazzo V, Pezzetti F, Scarano A, Piattelli A, Bignozzi CA, Massari L, Brunelli G, Carinci F. Zirconium oxide coating improves implant osseointegration in vivo. Dent Mater. 2008; 24:357–361.19. Yang Y, Kim KH, Ong JL. A review on calcium phosphate coatings produced using a sputtering process--an alternative to plasma spraying. Biomaterials. 2005; 26:327–337.20. Nelea V, Morosanu C, Iliescu M, Mihilescu IN. Microstructure and mechanical properties of hydroxyapatite thin films grown by RF magnetron sputtering. Surf Coat Technol. 2003; 173:315–322.21. Wan T, Aoki H, Hikawa J, Lee JH. RF-magnetron sputtering technique for producing hydroxyapatite coating film on various substrates. Biomed Mater Eng. 2007; 17:291–297.22. van Oss CJ, Chaudhury MK, Good RJ. Monopolar surfaces. Adv Colloid Interface Sci. 1987; 28:35–64.23. Rajaraman R, Rounds DE, Yen SP, Rembaum A. A scanning electron microscope study of cell adhesion and spreading in vitro. Exp Cell Res. 1974; 88:327–339.24. Hanawa T, Sakamoto H, Tanaka Y. Biofunctional hybride of titanium with polymers. Mater Sci Forum. 2007; 539-543:563–566.25. Kawahara H, Soeda Y, Niwa K, Takahashi M, Kawahara D, Araki N. In vitro study on bone formation and surface topography from the standpoint of biomechanics. J Mater Sci Mater Med. 2004; 15:1297–1307.26. Lincks J, Boyan BD, Blanchard CR, Lohmann CH, Liu Y, Cochran DL, Dean DD, Schwartz Z. Response of MG63 osteoblast-like cells to titanium and titanium alloy is dependent on surface roughness and composition. Biomaterials. 1998; 19:2219–2232.27. Kawahara H, Soeda Y, Niwa K, Takahashi M, Kawahara D, Araki N. In vitro study on bone formation and surface topography from the standpoint of biomechanics. J Mater Sci Mater Med. 2004; 15:1297–1307.28. Pessková V, Kubies D, Hulejová H, Himmlová L. The influence of implant surface properties on cell adhesion and proliferation. J Mater Sci Mater Med. 2007; 18:465–473.29. Gerstenfeld LC, Chipman SD, Glowacki J, Lian JB. Expression of differentiated function by mineralizing cultures of chicken osteoblasts. Dev Biol. 1987; 122:49–60.30. Ganss B, Kim RH, Sodek J. Bone sialoprotein. Crit Rev Oral Biol Med. 1999; 10:79–98.31. Hunter GK, Goldberg HA. Nucleation of hydroxyapatite by bone sialoprotein. Proc Natl Acad Sci U S A. 1993; 90:8562–8565.32. Paredes R, Arriagada G, Cruzat F, Olate J, Van Wijnen A, Lian J, Stein G, Stein J, Montecino M. The Runx2 transcription factor plays a key role in the 1alpha,25-dihydroxy Vitamin D3-dependent upregulation of the rat osteocalcin (OC) gene expression in osteoblastic cells. J Steroid Biochem Mol Biol. 2004; 89-90:269–271.33. Bacakova L, Filova E, Parizek M, Ruml T, Svorcik V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol Adv. 2011; 29:739–767.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of Zirconium Nitride coating on shear bond strength with denture base resin in Co-Cr alloy and titanium alloy
- Brazing characteristics of ZrO2 and Ti-6Al-4V brazed joints with increasing temperature
- Comparison of Cytocompatibility Between Grit Blasted Titanium Alloy (Ti-6Al-4V) with or without Pure Titanium Coating
- The evaluation of cytotoxicity and biocompatibility of Ti-Ta-Nb-base alloy
- Cell response to a newly developed Ti-10Ta-10Nb alloy and its sputtered nanoscale coating