J Adv Prosthodont.
2009 Mar;1(1):56-61. 10.4047/jap.2009.1.1.56.
Cell response to a newly developed Ti-10Ta-10Nb alloy and its sputtered nanoscale coating
- Affiliations
-
- 1Department of Prosthodontics, Graduate School, Chonnam National University, Gwang-Ju, Korea. psw320@chonnam.ac.kr
- KMID: 2176346
- DOI: http://doi.org/10.4047/jap.2009.1.1.56
Abstract
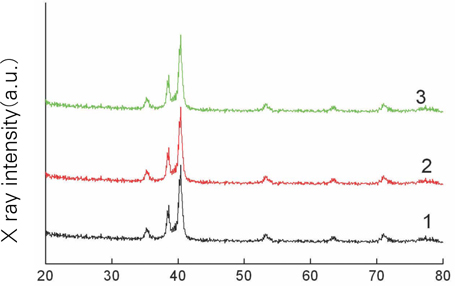
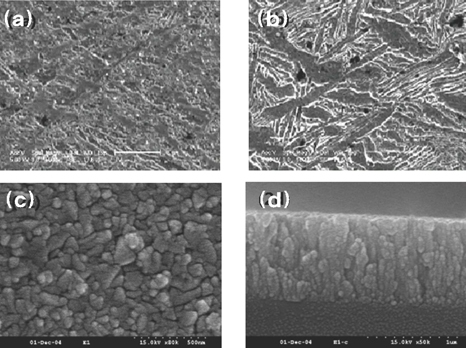
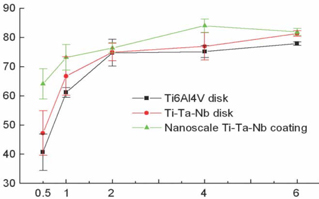
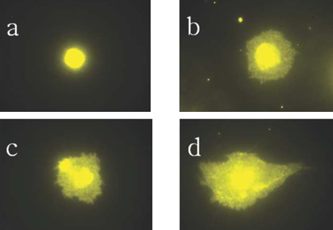
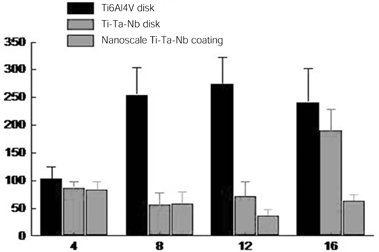
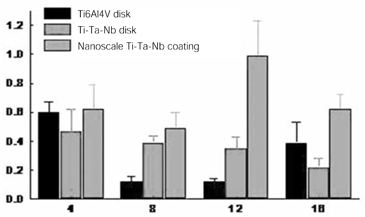
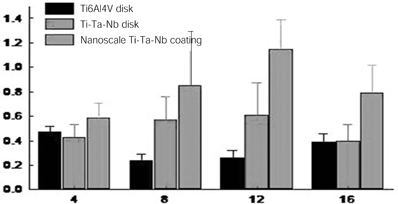
- STATEMENT OF PROBLEM: The success of titanium implants is due to osseointegration or the direct contact of the implant surface and bone without a fibrous connective tissue interface. PURPOSE: The purpose of this study was to evaluate the osteoblast precursor response to titanium - 10 tantalum - 10 niobium (Ti-Ta-Nb) alloy and its sputtered coating. MATERIAL AND METHODS: Ti-Ta-Nb coatings were sputtered onto the Ti-Ta-Nb disks. Ti6-Al-4V alloy disks were used as controls. An osteoblast precursor cell line, were used to evaluate the cell responses to the 3 groups. Cell attachment was measured using coulter counter and the cell morphology during attachment period was observed using fluorescent microscopy. Cell culture was performed at 4, 8, 12 and 16 days. RESULTS: The sputtered Ti-Ta-Nb coatings consisted of dense nanoscale grains in the range of 30 to 100 nm with alpha-Ti crystal structure. The Ti-Ta-Nb disks and its sputtered nanoscale coatings exhibited greater hydrophilicity and rougher surfaces compared to the Ti-6Al-4V disks. The sputtered nanoscale Ti-Ta-Nb coatings exhibited significantly greater cell attachment compared to Ti-6Al-4V and Ti-Ta-Nb disks. Nanoscale Ti-Ta-Nb coatings exhibited significantly greater ALP specific activity and total protein production compared to the other 2 groups. CONCLUSIONS: It was concluded that nanoscale Ti-Ta-Nb coatings enhance cell adhesion. In addition, Ti-Ta-Nb alloy and its nanoscale coatings enhanced osteoblast differentiation, but did not support osteoblast precursor proliferation compared to Ti-6Al-4V. These results indicate that the new developed Ti-Ta-Nb alloy and its nanoscale Ti-Ta-Nb coatings may be useful as an implant material.
Keyword
MeSH Terms
Figure
Reference
-
1. Albrektsson T, Jacobsson M. Bone-metal interface in osseointegration. J Prosthet Dent. 1987. 57:597–607.2. Kasemo B. Biocompatibility of titanium implants: surface science aspects. J Prosthet Dent. 1983. 49:832–837.3. Linter CM. Neuropsychiatric aspects of trace elements. Br J Hosp Med. 1985. 34:361–365.4. Srinivasan DP. Trace elements in psychiatric illness. Br J Hosp Med. 1984. 32:77–79.5. Levine S. The management of resistant depression. Acta Psychiatr Belg. 1986. 86:141–151.6. Li SJ, Niinomi M, Akahori T, Kasuga T, Yang R, Hao YL. Fatigue characteristics of bioactive glass-ceramic-coated Ti-29Nb-13Ta-4.6Zr for biomedical application. Biomaterials. 2004. 25:3369–3378.7. Niinomi M. Fatigue performance and cyto-toxicity of low rigidity titanium alloy, Ti-29Nb-13Ta-4.6Zr. Biomaterials. 2003. 24:2673–2683.8. Black J. Biological performance of tantalum. Clin Mater. 1994. 16:167–173.9. Meenaghan MA, Natiella JR, Moresi JL, Flynn HE, Wirth JE, Baier RE. Tissue response to surface-treated tantalum implants: preliminary observations in primates. J Biomed Mater Res. 1979. 13:631–643.10. Cohen R. A porous tantalum trabecular metal: basic science. Am J Orthop. 2002. 31:216–217.11. Limberger F, Lenz E. Biological evaluation of In-Ceram-ceramics compared to cobalt-base-alloys and the metals titanium, tantalum and niobium in animal experiments. Dtsch Stomatol. 1991. 41:407–410. [Article in German].12. Johansson CB, Hansson HA, Albrektsson T. Qualitative interfacial study between bone and tantalum, niobium or commercially pure titanium. Biomaterials. 1990. 11:277–280.13. Breme J, Wadewitz V. Comparison of titanium-tantalum and titanium-niobium alloys for application as dental implants. Int J Oral Maxillofac Implants. 1989. 4:113–118.14. Zitter H, Plenk H Jr. The electrochemical behavior of metallic implant materials as an indicator of their biocompatibility. J Biomed Mater Res. 1987. 21:881–896.15. Rabenseifner L, Küsswetter W, Wünsch PH, Schwab M. Is fracture healing in the presence of biocompatible implant materials tantalum and niobium different in comparison to steel implants? Z Orthop Ihre Grenzgeb. 1984. 122:349–355. [Article in German].16. Fontaine AB, Koelling K, Passos SD, Cearlock J, Hoffman R, Spigos DG. Polymeric surface modifications of tantalum stents. J Endovasc Surg. 1996. 3:276–283.17. Tepe G, Duda SH, Hanke H, Schulze S, Hagmeier S, Bruck B, Schott U, Betz E, Schmahl FW, Claussen CD. Covered stents for prevention of restenosis. Experimental and clinical results with different stent designs. Invest Radiol. 1996. 31:223–229.18. Crochet D, Grossetête R, Bach-Lijour B, Sagan C, Lecomte E, Leurent B, Brunel P, Le Nihouannen JC. Plasma treatment effects on the tantalum Strecker stent implanted in femoral arteries of sheep. Cardiovasc Intervent Radiol. 1994. 17:285–291.19. Brumme S, Löwicke G, Knöfler W. The use of tantalum wire as a suture material. Z Exp Chir Transplant Kunstliche Organe. 1989. 22:308–313. [German].20. Shimko DA, Shimko VF, Sander EA, Dickson KF, Nauman EA. Effect of porosity on the fluid flow characteristics and mechanical properties of tantalum scaffolds. J Biomed Mater Res B Appl Biomater. 2005. 73:315–324.21. Zardiackas LD, Parsell DE, Dillon LD, Mitchell DW, Nunnery LA, Poggie R. Structure, metallurgy, and mechanical properties of a porous tantalum foam. J Biomed Mater Res. 2001. 58:180–187.22. Hacking SA, Bobyn JD, Toh K, Tanzer M, Krygier JJ. Fibrous tissue ingrowth and attachment to porous tantalum. J Biomed Mater Res. 2000. 52:631–638.23. Bagley J, Rosenzweig M, Marks DF, Pykett MJ. Extended culture of multipotent hematopoietic progenitors without cytokine augmentation in a novel three-dimensional device. Exp Hematol. 1999. 27:496–504.24. Webster TJ, Siegel RW, Bizios R. Osteoblast adhesion on nanophase ceramics. Biomaterials. 1999. 20:1221–1227.25. Shah AK, Sinha RK, Hickok NJ, Tuan RS. High-resolution morphometric analysis of human osteoblastic cell adhesion on clinically relevant orthopedic alloys. Bone. 1999. 24:499–506.26. Berube P, Yang Y, Carnes DL, Stover RE, Boland EJ, Ong JL. The effect of sputtered calcium phosphate coatings of different crystallinity on osteoblast differentiation. J Periodontol. 2005. 76:1697–1709.27. Kartsogiannis V, Ng KW. Cell lines and primary cell cultures in the study of bone cell biology. Mol Cell Endocrinol. 2004. 228:79–102.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The evaluation of cytotoxicity and biocompatibility of Ti-Ta-Nb-base alloy
- Osteoblastic behavior to zirconium coating on Ti-6Al-4V alloy
- The effect of Zirconium Nitride coating on shear bond strength with denture base resin in Co-Cr alloy and titanium alloy
- Comparison of Cytocompatibility Between Grit Blasted Titanium Alloy (Ti-6Al-4V) with or without Pure Titanium Coating
- Biological response of primary rat calvarial cell by surface treatment of Ti-8Ta-8Nb alloy