Ann Dermatol.
2013 Aug;25(3):315-320. 10.5021/ad.2013.25.3.315.
A Novel Compound Rasatiol Isolated from Raphanus sativus Has a Potential to Enhance Extracellular Matrix Synthesis in Dermal Fibroblasts
- Affiliations
-
- 1Department of Dermatology and Research Institute for Medical Sciences, School of Medicine, Chungnam National University, Daejeon, Korea. jhoon@cnu.ac.kr
- 2Department of Biotechnology, Hanbat National University, Daejeon, Korea.
- 3Oriental BioMed Lab, Daejeon, Korea.
- 4Oriental Medical Colleage of Daejeon University, Daejeon, Korea.
- KMID: 2265871
- DOI: http://doi.org/10.5021/ad.2013.25.3.315
Abstract
- BACKGROUND
The fibrous proteins of extracellular matrix (ECM) produced by dermal fibroblast contributes to the maintenance of connective tissue integrity.
OBJECTIVE
This study is carried out to identify the bioactive ingredient from natural products that enhances ECM production in dermal fibroblasts.
METHODS
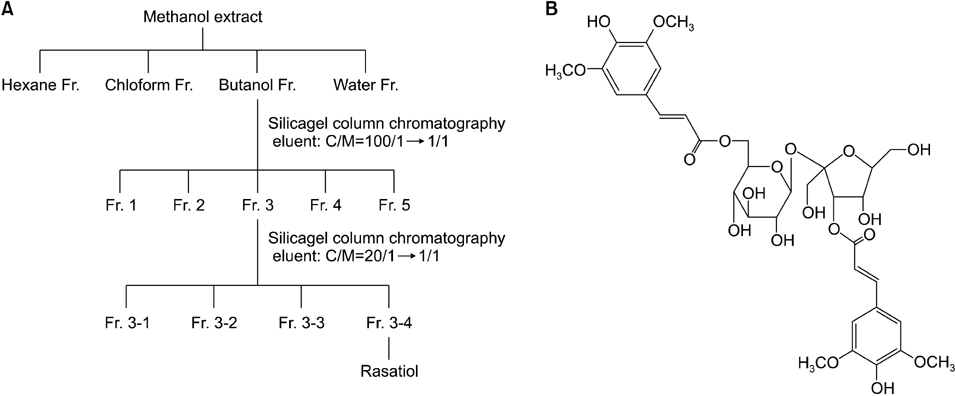
Bioassay-directed fractionation was used to isolate the active ingredient from natural extracts. The effects of rasatiol (isolated from Raphanus sativus) on ECM production in primary cultured human dermal fibroblasts was investigated by enzyme linked immunosorbent assay and western blot analysis.
RESULTS
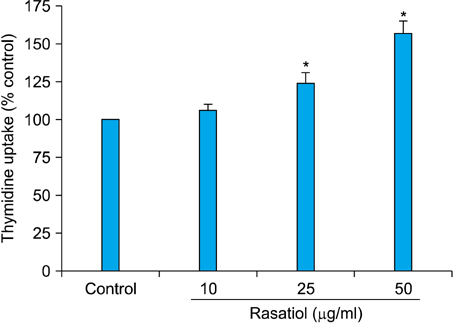
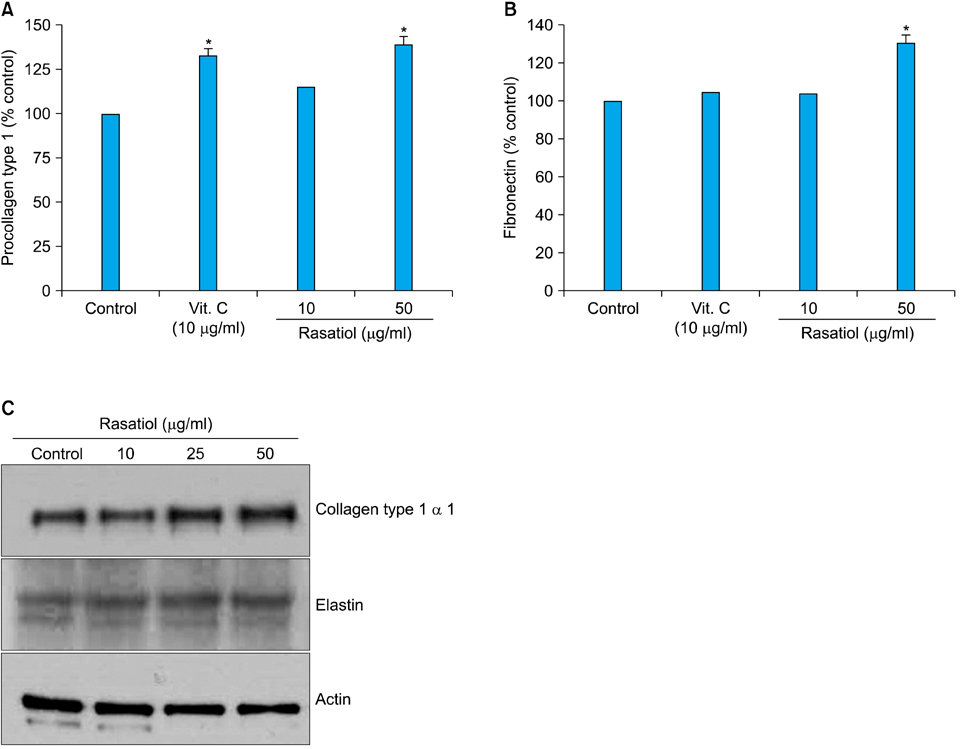
Rasatiol accelerated fibroblast growth in a dose-dependent manner and increased the production of type 1 collagen, fibronectin and elastin. Phosphorylation of p42/44 extracellular signal-regulated kinase, p38 mitogen-activated protein kinase, and Akt was remarkably increased by rasatiol, indicating that enhanced ECM production is linked to the activation of intracellular signaling cascades.
CONCLUSION
These results indicate that rasatiol stimulates the fibrous components of ECM production, and may be applied to the maintenance of skin texture.
Keyword
MeSH Terms
-
Biological Agents
Blotting, Western
Collagen Type I
Connective Tissue
Elastin
Enzyme-Linked Immunosorbent Assay
Extracellular Matrix
Fibroblasts
Fibronectins
Humans
Phosphorylation
Phosphotransferases
Protein Kinases
Raphanus
Scleroproteins
Skin
Biological Agents
Collagen Type I
Elastin
Fibronectins
Phosphotransferases
Protein Kinases
Scleroproteins
Figure
Reference
-
1. Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010; 123:4195–4200.
Article2. Pieraggi MT, Bouissou H, Angelier C, Uhart D, Magnol JP, Kokolo J. The fibroblast. Ann Pathol. 1985; 5:65–76.3. Uitto J. Connective tissue biochemistry of the aging dermis. Age-related alterations in collagen and elastin. Dermatol Clin. 1986; 4:433–446.4. Ignotz RA, Massagué J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986; 261:4337–4345.
Article5. Lim IJ, Phan TT, Tan EK, Nguyen TT, Tran E, Longaker MT, et al. Synchronous activation of ERK and phosphatidylinositol 3-kinase pathways is required for collagen and extracellular matrix production in keloids. J Biol Chem. 2003; 278:40851–40858.
Article6. Lee DJ, Rosenfeldt H, Grinnell F. Activation of ERK and p38 MAP kinases in human fibroblasts during collagen matrix contraction. Exp Cell Res. 2000; 257:190–197.
Article7. Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999; 96:857–868.
Article8. Stanikunaite R, Khan SI, Trappe JM, Ross SA. Cyclooxygenase-2 inhibitory and antioxidant compounds from the truffle Elaphomyces granulatus. Phytother Res. 2009; 23:575–578.
Article9. Itoh A, Isoda K, Kondoh M, Kawase M, Kobayashi M, Tamesada M, et al. Hepatoprotective effect of syringic acid and vanillic acid on concanavalin a-induced liver injury. Biol Pharm Bull. 2009; 32:1215–1219.
Article10. Yoon IK, Kim HK, Kim YK, Song IH, Kim W, Kim S, et al. Exploration of replicative senescence-associated genes in human dermal fibroblasts by cDNA microarray technology. Exp Gerontol. 2004; 39:1369–1378.
Article11. Cristofalo VJ, Pignolo RJ. Replicative senescence of human fibroblast-like cells in culture. Physiol Rev. 1993; 73:617–638.
Article12. Sephel GC, Davidson JM. Elastin production in human skin fibroblast cultures and its decline with age. J Invest Dermatol. 1986; 86:279–285.
Article13. Gildner CD, Lerner AL, Hocking DC. Fibronectin matrix polymerization increases tensile strength of model tissue. Am J Physiol Heart Circ Physiol. 2004; 287:H46–H53.
Article14. Nagai Y, Miyata K, Sun GP, Rahman M, Kimura S, Miyatake A, et al. Aldosterone stimulates collagen gene expression and synthesis via activation of ERK1/2 in rat renal fibroblasts. Hypertension. 2005; 46:1039–1045.
Article15. Sato M, Shegogue D, Gore EA, Smith EA, McDermott PJ, Trojanowska M. Role of p38 MAPK in transforming growth factor beta stimulation of collagen production by scleroderma and healthy dermal fibroblasts. J Invest Dermatol. 2002; 118:704–711.
Article16. Rodríguez-Barbero A, Obreo J, Yuste L, Montero JC, Rodríguez-Peña A, Pandiella A, et al. Transforming growth factor-beta1 induces collagen synthesis and accumulation via p38 mitogen-activated protein kinase (MAPK) pathway in cultured L(6)E(9) myoblasts. FEBS Lett. 2002; 513:282–288.17. Bujor AM, Pannu J, Bu S, Smith EA, Muise-Helmericks RC, Trojanowska M. Akt blockade downregulates collagen and upregulates MMP1 in human dermal fibroblasts. J Invest Dermatol. 2008; 128:1906–1914.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Oncostatin M on Wound Healing Activity of Diabetic Fibroblasts in vitro
- Behavior of Fibroblasts on a Porous Hyaluronic Acid Incorporated Collagen Matrix
- Effect of Estrogen on Fibroblast Proliferation and Collagen Synthesis
- Molecular Mechanisms and In Vivo Mouse Models of Skin Aging Associated with Dermal Matrix Alterations
- Effect of Oncostatin M on Proliferation and Matrix Synthesis of Dermal Fibroblasts