Ann Dermatol.
2010 May;22(2):173-179. 10.5021/ad.2010.22.2.173.
Stimulation of the Extracellular Matrix Production in Dermal Fibroblasts by Velvet Antler Extract
- Affiliations
-
- 1Department of Dermatology and the Research Institute for Medical Sciences, School of Medicine, Chungnam National University, Daejeon, Korea. jhoon@cnu.ac.kr
- 2Oriental BioMed Lab, Daejeon, Korea.
- 3LG Household & Personal Care Ltd, Daejeon, Korea.
- 4Department of Dermatology and the Institute of Health Sciences, School of Medicine, Gyeongsang National University, Jinju, Korea.
- KMID: 2265388
- DOI: http://doi.org/10.5021/ad.2010.22.2.173
Abstract
- BACKGROUND
Fibroblasts produce many components of the extracellular matrix (ECM) and so they contribute to the maintenance of connective tissue integrity.
OBJECTIVE
The aim of this study is to evaluate the effect of velvet antler extract (VAE) on the ECM production of dermal fibroblasts cultured in vitro.
METHODS
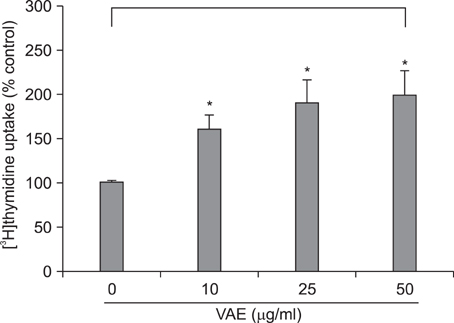
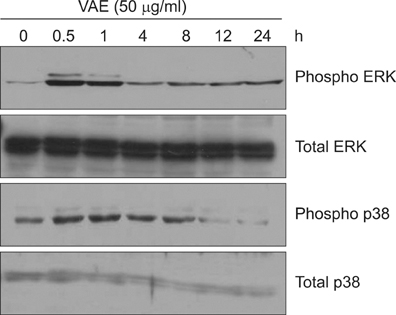
Primary cultured human dermal fibroblasts were treated with VAE, and then the ECM production was determined by RT-PCR, ELISA and Western blot analysis. Furthermore, the change of gene expression according to VAE treatment was evaluated by cDNA microarray. RESULTS: VAE accelerated the growth of fibroblasts in a dose-dependent manner. VAE increased the production of several ECM components, including type 1 collagen, fibronectin and elastin. In line with these results, the phosphorylations of p42/44 ERK and p38 mitogen-activated protein kinase were markedly increased by VAE, suggesting that the enhancement of ECM production may be linked to the activation of intracellular signaling cascades. VAE also significantly increased cell migration on an in vitro scratch wound test. In cDNA microarray, many genes related with connective tissue integrity were identified to be up-regulated by VAE.
CONCLUSION
These results suggest that VAE has a potential to stimulate ECM production, and VAE may be applicable for maintaining the skin's texture.
MeSH Terms
Figure
Cited by 2 articles
-
Cedrol Enhances Extracellular Matrix Production in Dermal Fibroblasts in a MAPK-Dependent Manner
Mu Hyun Jin, Sun Gyoo Park, Yul-Lye Hwang, Min-Ho Lee, Nam-Ji Jeong, Seok-Seon Roh, Young Lee, Chang Deok Kim, Jeung-Hoon Lee
Ann Dermatol. 2012;24(1):16-21. doi: 10.5021/ad.2012.24.1.16.The Effect of Rhus verniciflua Stokes Extracts on Photo-Aged Mouse Skin
Hannah Hong, Minyoung Jung, Sung Jay Choe, Jung-Bae Kim, Eung Ho Choi
Ann Dermatol. 2017;29(3):295-301. doi: 10.5021/ad.2017.29.3.295.
Reference
-
1. Pieraggi MT, Bouissou H, Angelier C, Uhart D, Magnol JP, Kokolo J. The fibroblast. Ann Pathol. 1985. 5:65–76.2. Uitto J. Connective tissue biochemistry of the aging dermis. Age-related alterations in collagen and elastin. Dermatol Clin. 1986. 4:433–446.3. Chan JC, Duszczyszyn DA, Castellino FJ, Ploplis VA. Accelerated skin wound healing in plasminogen activator inhibitor-1-deficient mice. Am J Pathol. 2001. 159:1681–1688.
Article4. Ignotz RA, Massague J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986. 261:4337–4345.
Article5. Jhon GJ, Park SY, Han SY, Lee S, Kim Y, Chang YS. Studies of the chemical structure of gangliosides in deer antler, Cervus nippon. Chem Pharm Bull (Tokyo). 1999. 47:123–127.
Article6. Kang SK, Kim KS, Kim SI, Chung KH, Lee IS, Kim CH. Immunosuppressive activity of deer antler extracts of Cervus korean TEMMINCK var. mantchuricus Swinhoe, on type II collagen-induced arthritis. In Vitro Cell Dev Biol Anim. 2006. 42:100–107.
Article7. Li YJ, Kim TH, Kwak HB, Lee ZH, Lee SY, Jhon GJ. Chloroform extract of deer antler inhibits osteoclast differentiation and bone resorption. J Ethnopharmacol. 2007. 113:191–198.
Article8. Yang HO, Kim SH, Cho SH, Kim MG, Seo JY, Park JS, et al. Purification and structural determination of hematopoietic stem cell-stimulating monoacetyldiglycerides from Cervus nippon (deer antler). Chem Pharm Bull (Tokyo). 2004. 52:874–878.
Article9. Guan SW, Duan LX, Li YY, Wang BX, Zhou QL. A novel polypeptide from Cervus nippon Temminck proliferation of epidermal cells and NIH3T3 cell line. Acta Biochim Pol. 2006. 53:395–397.
Article10. Mikler JR, Theoret CL, High JC. Effects of topical elk velvet antler on cutaneous wound healing in streptozotocin-induced diabetic rats. J Altern Complement Med. 2004. 10:835–840.
Article11. Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007. 2:329–333.
Article12. Lim IJ, Phan TT, Tan EK, Nguyen TT, Tran E, Longaker MT, et al. Synchronous activation of ERK and phosphatidylinositol 3-kinase pathways is required for collagen and extracellular matrix production in keloids. J Biol Chem. 2003. 278:40851–40858.
Article13. Lee DJ, Rosenfeldt H, Grinnell F. Activation of ERK and p38 MAP kinases in human fibroblasts during collagen matrix contraction. Exp Cell Res. 2000. 257:190–197.
Article14. Kanzaki T, Morisaki N, Shiina R, Saito Y. Role of transforming growth factor-beta pathway in the mechanism of wound healing by saponin from Ginseng Radix rubra. Br J Pharmacol. 1998. 125:255–262.
Article15. Quan T, He T, Kang S, Voorhees JJ, Fisher GJ. Solar ultraviolet irradiation reduces collagen in photoaged human skin by blocking transforming growth factor-beta type II receptor/Smad signaling. Am J Pathol. 2004. 165:741–751.
Article16. Sephel GC, Davidson JM. Elastin production in human skin fibroblast cultures and its decline with age. J Invest Dermatol. 1986. 86:279–285.
Article17. Falanga V, Isaacs C, Paquette D, Downing G, Kouttab N, Butmarc J, et al. Wounding of bioengineered skin: cellular and molecular aspects after injury. J Invest Dermatol. 2002. 119:653–660.
Article18. Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997. 18:4–25.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective actions of Rubus coreanus ethanol extract on collagenous extracellular matrix in ultraviolet-B irradiation-induced human dermal fibroblasts
- Cedrol Enhances Extracellular Matrix Production in Dermal Fibroblasts in a MAPK-Dependent Manner
- A Novel Compound Rasatiol Isolated from Raphanus sativus Has a Potential to Enhance Extracellular Matrix Synthesis in Dermal Fibroblasts
- Inhibitory effect of Aralia elata ethanol extract against skin damage in UVB-exposed human keratinocytes and human dermal fibroblasts
- Molecular Mechanisms and In Vivo Mouse Models of Skin Aging Associated with Dermal Matrix Alterations