Ann Dermatol.
2014 Dec;26(6):681-687. 10.5021/ad.2014.26.6.681.
Superoxide Dismutase 1 Inhibits Alpha-Melanocyte Stimulating Hormone and Ultraviolet B-Induced Melanogenesis in Murine Skin
- Affiliations
-
- 1Department of Dermatology, Chung-Ang University College of Medicine, Seoul, Korea. beomjoon@unitel.co.kr
- 2Nutrex Technology R&D Center, Seoul, Korea.
- 3Biomedical Research Institute, MEDIPOST Co., Ltd., Seongnam, Korea.
- 4Department of Medicine, Graduate School, Chung-Ang University, Seoul, Korea.
- 5MB Business Development Team, Pacificpharma, Seoul, Korea.
- 6Cosmeceutical Team, Pacificpharma, Seoul, Korea.
- KMID: 2264861
- DOI: http://doi.org/10.5021/ad.2014.26.6.681
Abstract
- BACKGROUND
Over the last decade, the incidence of ultraviolet B (UVB)-related skin problems has increased. Oxidative stress caused by UVB induces the secretion of melanocyte growth and activating factors from keratinocytes, which results in the formation of cutaneous hyperpigmentation. Therefore, increasing the antioxidant abilities of skin cells is thought to be a beneficial strategy for the development of sunscreen agents. Superoxide dismutase 1 (SOD1) is an antioxidant enzyme that is known to exhibit antioxidant properties.
OBJECTIVE
The purpose of this study was to investigate the effect of SOD1 on alpha-melanocyte stimulating hormone (alpha-MSH) and UVB-induced melanogenesis in B16F10 melanoma cells and HRM-2 melanin-possessing hairless mice.
METHODS
The inhibitory effect of SOD1 on tyrosinase activity was evaluated in a cell-free system. Additional experiments were performed using B16F10 melanoma cells to demonstrate the effects of SOD1 in vitro, and HRM-2 melanin-possessing hairless mice were used to evaluate the antimelanogenic effects of SOD1 in vivo.
RESULTS
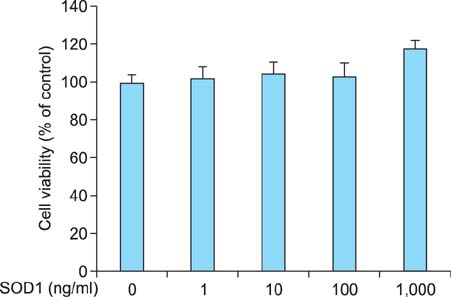
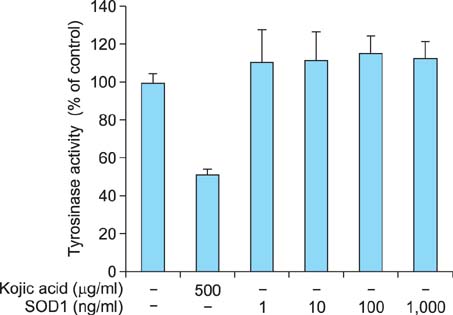
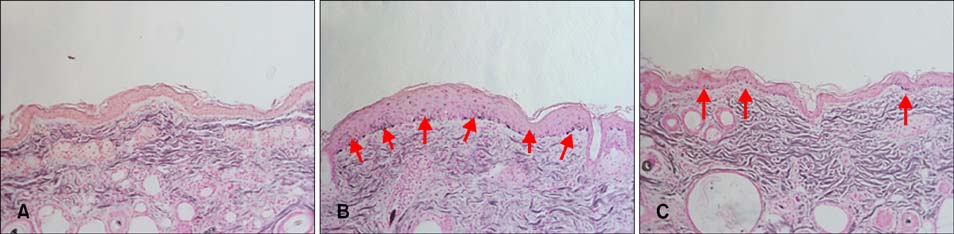
We found that SOD1 inhibited melanin production in a dose-dependent manner without causing cytotoxicity in B16F10 melanoma cells. SOD1 did not inhibit tyrosinase activity under cell-free conditions. The results indicate that SOD1 may reduce pigmentation by an indirect, nonenzymatic mechanism. We also found that SOD1 decreased UVB-induced melanogenesis in HRM-2 melanin-possessing hairless mice, as visualized through hematoxylin and eosin staining and Fontana-Masson staining.
CONCLUSION
Our results indicate that SOD1 has an inhibitory effect on alpha-MSH and UVB-induced melanogenesis, indicating that SOD1 may be a promising sunscreen agent.
MeSH Terms
-
alpha-MSH
Animals
Cell-Free System
Eosine Yellowish-(YS)
Hematoxylin
Hyperpigmentation
Incidence
Keratinocytes
Melanins
Melanocytes
Melanoma
Mice
Mice, Hairless
Monophenol Monooxygenase
Oxidative Stress
Pigmentation
Skin Pigmentation
Skin*
Superoxide Dismutase*
Eosine Yellowish-(YS)
Hematoxylin
Melanins
Monophenol Monooxygenase
Superoxide Dismutase
alpha-MSH
Figure
Reference
-
1. Nole G, Johnson AW. An analysis of cumulative lifetime solar ultraviolet radiation exposure and the benefits of daily sun protection. Dermatol Ther. 2004; 17:Suppl 1. 57–62.
Article2. Gonzalez Maglio DH, Paz ML, Ferrari A, Weill FS, Czerniczyniec A, Leoni J, et al. Skin damage and mitochondrial dysfunction after acute ultraviolet B irradiation: relationship with nitric oxide production. Photodermatol Photoimmunol Photomed. 2005; 21:311–317.
Article3. Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004; 195:298–308.
Article4. Gloster HM Jr, Brodland DG. The epidemiology of skin cancer. Dermatol Surg. 1996; 22:217–226.
Article5. Fisher MS, Kripke ML. Suppressor T lymphocytes control the development of primary skin cancers in ultraviolet-irradiated mice. Science. 1982; 216:1133–1134.
Article6. Ullrich SE. Mechanisms underlying UV-induced immune suppression. Mutat Res. 2005; 571:185–205.
Article7. Mørch CD, Gazerani P, Nielsen TA, Arendt-Nielsen L. The UVB cutaneous inflammatory pain model: a reproducibility study in healthy volunteers. Int J Physiol Pathophysiol Pharmacol. 2013; 5:203–215.8. Friedmann PS, Gilchrest BA. Ultraviolet radiation directly induces pigment production by cultured human melanocytes. J Cell Physiol. 1987; 133:88–94.
Article9. Gilchrest BA. A review of skin ageing and its medical therapy. Br J Dermatol. 1996; 135:867–875.
Article10. Kadekaro AL, Kavanagh RJ, Wakamatsu K, Ito S, Pipitone MA, Abdel-Malek ZA. Cutaneous photobiology. The melanocyte vs. the sun: who will win the final round? Pigment Cell Res. 2003; 16:434–447.
Article11. Meredith P, Sarna T. The physical and chemical properties of eumelanin. Pigment Cell Res. 2006; 19:572–594.
Article12. Hill HZ, Hill GJ. UVA, pheomelanin and the carcinogenesis of melanoma. Pigment Cell Res. 2000; 13:Suppl 8. 140–144.
Article13. Rouzaud F, Kadekaro AL, Abdel-Malek ZA, Hearing VJ. MC1R and the response of melanocytes to ultraviolet radiation. Mutat Res. 2005; 571:133–152.
Article14. Yamaguchi Y, Takahashi K, Zmudzka BZ, Kornhauser A, Miller SA, Tadokoro T, et al. Human skin responses to UV radiation: pigment in the upper epidermis protects against DNA damage in the lower epidermis and facilitates apoptosis. FASEB J. 2006; 20:1486–1488.
Article15. Miyamura Y, Coelho SG, Wolber R, Miller SA, Wakamatsu K, Zmudzka BZ, et al. Regulation of human skin pigmentation and responses to ultraviolet radiation. Pigment Cell Res. 2007; 20:2–13.
Article16. Svobodová A, Zdarilová A, Malisková J, Mikulková H, Walterová D, Vostalová J. Attenuation of UVA-induced damage to human keratinocytes by silymarin. J Dermatol Sci. 2007; 46:21–30.
Article17. Tobi SE, Gilbert M, Paul N, McMillan TJ. The green tea polyphenol, epigallocatechin-3-gallate, protects against the oxidative cellular and genotoxic damage of UVA radiation. Int J Cancer. 2002; 102:439–444.
Article18. Eller MS, Ostrom K, Gilchrest BA. DNA damage enhances melanogenesis. Proc Natl Acad Sci U S A. 1996; 93:1087–1092.
Article19. Halaban R, Pomerantz SH, Marshall S, Lerner AB. Tyrosinase activity and abundance in Cloudman melanoma cells. Arch Biochem Biophys. 1984; 230:383–387.
Article20. Hunt G, Kyne S, Wakamatsu K, Ito S, Thody AJ. Nle4DPhe7 alpha-melanocyte-stimulating hormone increases the eumelanin: phaeomelanin ratio in cultured human melanocytes. J Invest Dermatol. 1995; 104:83–85.
Article21. Wong G, Pawelek J. Melanocyte-stimulating hormone promotes activation of pre-existing tyrosinase molecules in Cloudman S91 melanoma cells. Nature. 1975; 255:644–646.
Article22. Matsuda H, Higashino M, Nakai Y, Iinuma M, Kubo M, Lang FA. Studies of cuticle drugs from natural sources. IV. Inhibitory effects of some Arctostaphylos plants on melanin biosynthesis. Biol Pharm Bull. 1996; 19:153–156.
Article23. Tobin D, Thody AJ. The superoxide anion may mediate short- but not long-term effects of ultraviolet radiation on melanogenesis. Exp Dermatol. 1994; 3:99–105.
Article24. Chung KW, Park YJ, Choi YJ, Park MH, Ha YM, Uehara Y, et al. Evaluation of in vitro and in vivo anti-melanogenic activity of a newly synthesized strong tyrosinase inhibitor (E)-3-(2,4 dihydroxybenzylidene)pyrrolidine-2,5-dione (3-DBP). Biochim Biophys Acta. 2012; 1820:962–969.
Article25. Song K, An SM, Kim M, Koh JS, Boo YC. Comparison of the antimelanogenic effects of p-coumaric acid and its methyl ester and their skin permeabilities. J Dermatol Sci. 2011; 63:17–22.
Article26. Pence BC, Naylor MF. Effects of single-dose ultraviolet radiation on skin superoxide dismutase, catalase, and xanthine oxidase in hairless mice. J Invest Dermatol. 1990; 95:213–216.
Article27. Okada K, Takahashi Y, Ohnishi K, Ishikawa O, Miyachi Y. Time-dependent effect of chronic UV irradiation on superoxide dismutase and catalase activity in hairless mice skin. J Dermatol Sci. 1994; 8:183–186.
Article28. Kvam E, Dahle J. Pigmented melanocytes are protected against ultraviolet-A-induced membrane damage. J Invest Dermatol. 2003; 121:564–569.
Article29. Zbytek B, Carlson JA, Granese J, Ross J, Mihm MC Jr, Slominski A. Current concepts of metastasis in melanoma. Expert Rev Dermatol. 2008; 3:569–585.
Article30. Peng LH, Liu S, Xu SY, Chen L, Shan YH, Wei W, et al. Inhibitory effects of salidroside and paeonol on tyrosinase activity and melanin synthesis in mouse B16F10 melanoma cells and ultraviolet B-induced pigmentation in guinea pig skin. Phytomedicine. 2013; 20:1082–1087.
Article31. Kumar KJ, Vani MG, Wang SY, Liao JW, Hsu LS, Yang HL, et al. in vitro and in vivo studies disclosed the depigmenting effects of gallic acid: a novel skin lightening agent for hyperpigmentary skin diseases. Biofactors. 2013; 39:259–270.
Article32. Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995; 80:179–185.
Article33. Lei TC, Virador VM, Vieira WD, Hearing VJ. A melanocytekeratinocyte coculture model to assess regulators of pigmentation in vitro. Anal Biochem. 2002; 305:260–268.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The change of superoxide dismutase activity in mouse skin by ultraviolet radiation
- Baicalein Inhibits αα-Melanocyte-stimulating Hormonestimulated Melanogenesis via p38 Mitogen-activated Protein Kinase Pathway in B16F10 Mouse Melanoma Cells
- Inhibitory Effects of Resveratrol on Melanin Synthesis in Ultraviolet B-Induced Pigmentation in Guinea Pig Skin
- A Study on the Effect of Superoxide Dismutase to Sunburn Cell Production in Mouse Skin By Ultraviolet Irradiation
- Inhibitory effect of Gastrodia elata Blume extract on alpha-melanocyte stimulating hormone-induced melanogenesis in murine B16F10 melanoma