Nutr Res Pract.
2017 Jun;11(3):173-179. 10.4162/nrp.2017.11.3.173.
Inhibitory effect of Gastrodia elata Blume extract on alpha-melanocyte stimulating hormone-induced melanogenesis in murine B16F10 melanoma
- Affiliations
-
- 1Department of Food and Nutrition, Soongeui Women's College, Seoul 04628, Korea.
- 2Department of Food and Nutrition, College of Natural Sciences, Myongji University, 116 Myongji-ro, Cheoin-gu, Yongin, Gyeonggi 17058, Korea. jhwang@mju.ac.kr
- 3BK Bio Co. Ltd., Seongnam, Gyeonggi 13229, Korea.
- KMID: 2407764
- DOI: http://doi.org/10.4162/nrp.2017.11.3.173
Abstract
- BACKGROUND/OBJECTIVES
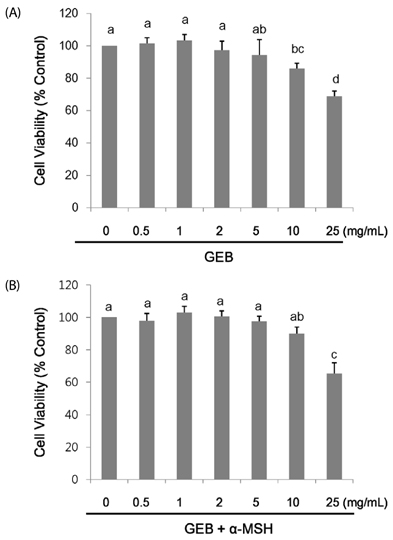
Gastrodia elata Blume (GEB), a traditional herbal medicine, has been used to treat a wide range of neurological disorders (e.g., paralysis and stroke) and skin problems (e.g., atopic dermatitis and eczema) in oriental medicine. This study was designed to investigate whether GEB extract inhibits melanogenesis activity in murine B16F10 melanoma. MATERIALS/METHOD: Murine B16F10 cells were treated with 0-5 mg/mL of GEB extract or 400 µg/mL arbutin (a positive control) for 72 h after treatment with/without 200 nM alpha-melanocyte stimulating hormone (α-MSH) for 24 h. Melanin concentration, tyrosinase activity, mRNA levels, and protein expression of microphthalmia-associated transcription factor (MITF), tyrosinase, tyrosinase-related protein (Trp)1, and Trp2 were analyzed in α-MSH-untreated and α-MSH-treated B16F10 cells.
RESULTS
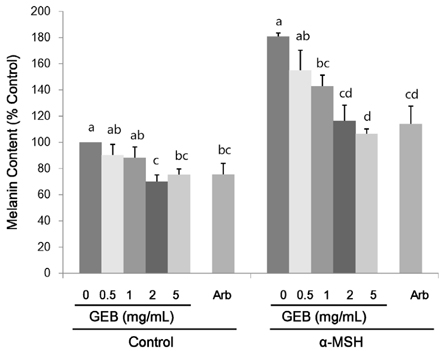
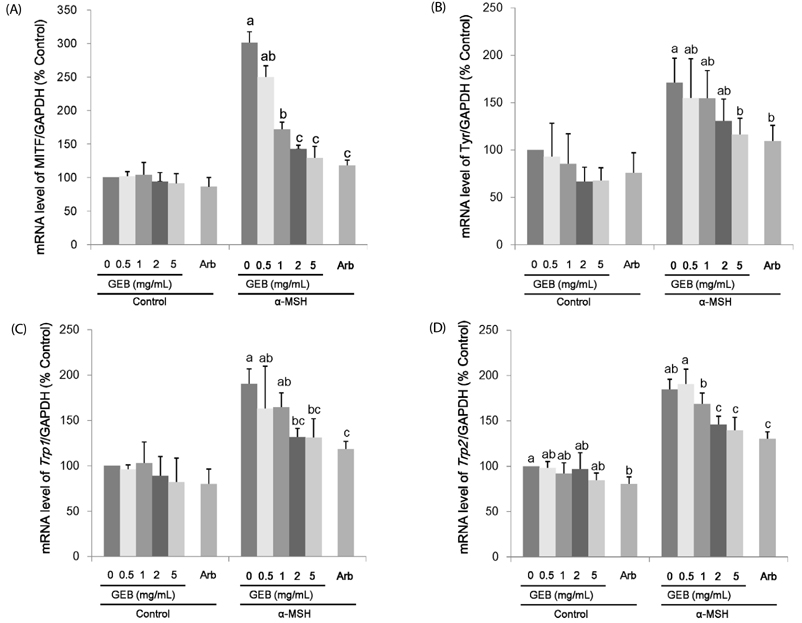
Treatment with 200 nM α-MSH induced almost 2-fold melanin synthesis and tyrosinase activity along with increased mRNA levels and protein expression of MITF, tyrosinase, Trp1 and Trp2. Irrespective of α-MSH stimulation, GEB extract at doses of 0.5-5 mg/mL inhibited all these markers for skin whitening in a dose-dependent manner. While lower doses (0.5-1 mg/mL) of GEB extract generally had a tendency to decrease melanogenesis, tyrosinase activity, and mRNA levels and protein expression of MITF, tyrosinase, Trp1, and Trp2, higher doses (2-5 mg/mL) significantly inhibited all these markers in α-MSH-treated B16F10 cells in a dose-dependent manner. These inhibitory effects of the GEB extract at higher concentrations were similar to those of 400 µg/mL arbutin, a well-known depigmenting agent.
CONCLUSIONS
These results suggest that GEB displays dose-dependent inhibition of melanin synthesis through the suppression of tyrosinase activity as well as molecular levels of MITF, tyrosinase, Trp1, and Trp2 in murine B16F10 melanoma. Therefore, GEB may be an effective and natural skin-whitening agent for application in the cosmetic industry.
Keyword
MeSH Terms
-
Arbutin
Dermatitis, Atopic
Gastrodia*
Herbal Medicine
Medicine, East Asian Traditional
Melanins
Melanoma*
Microphthalmia-Associated Transcription Factor
Monophenol Monooxygenase
Nervous System Diseases
Paralysis
RNA, Messenger
Skin
Skin Lightening Preparations
Arbutin
Melanins
Microphthalmia-Associated Transcription Factor
Monophenol Monooxygenase
RNA, Messenger
Skin Lightening Preparations
Figure
Cited by 1 articles
-
Anti-melanogenic effects of Hordeum vulgare L. barely sprout extract in murine B16F10 melanoma cells
Jeong-Hwa Choi, Jong-Gi Jung, Jung-Eun Kim, Mi-Ae Bang
J Nutr Health. 2019;52(2):168-175. doi: 10.4163/jnh.2019.52.2.168.
Reference
-
1. Chang T. Natural melanogenesis inhibitors acting through the down-regulation of tyrosinase activity. c. 2012; 5:1661–1685.
Article2. Chen WC, Tseng TS, Hsiao NW, Lin YL, Wen ZH, Tsai CC, Lee YC, Lin HH, Tsai KC. Discovery of highly potent tyrosinase inhibitor, T1, with significant anti-melanogenesis ability by zebrafish in vivo assay and computational molecular modeling. Sci Rep. 2015; 5:7995–8002.
Article3. Arndt KA, Fitzpatrick TB. Topical use of hydroquinone as a depigmenting agent. JAMA. 1965; 194:965–967.
Article4. Heilgemeir GP, Balda BR. Irreversible toxic depigmentation. Observations following use of hydroquinonemonobenzylether-containing skin bleaching preparations. MMW Munch Med Wochenschr. 1981; 123:47–48.5. Mishima Y, Hatta S, Ohyama Y, Inazu M. Induction of melanogenesis suppression: cellular pharmacology and mode of differential action. Pigment Cell Res. 1988; 1:367–374.
Article6. Ros JR, Rodríguez-López JN, García-Cánovas F. Effect of L-ascorbic acid on the monophenolase activity of tyrosinase. Biochem J. 1993; 295:309–312.
Article7. Funayama M, Arakawa H, Yamamoto R, Nishino T, Shin T, Murao S. Effects of α-and β-arbutin on activity of tyrosinases from mushroom and mouse melanoma. Biosci Biotechnol Biochem. 1995; 59:143–144.
Article8. Park E, Lee WY, Kim ST, Ahn JK, Bae EK. Ergothioneine accumulation in a medicinal plant Gastrodia elata. J Med Plants Res. 2010; 4:1141–1147.9. Jang JH, Son Y, Kang SS, Bae CS, Kim JC, Kim SH, Shin T, Moon C. Neuropharmacological potential of Gastrodia elata Blume and its components. Evid Based Complement Alternat Med. 2015; 2015:309261.10. Chik SC, Or TC, Luo D, Yang CL, Lau AS. Pharmacological effects of active compounds on neurodegenerative disease with gastrodia and uncaria decoction, a commonly used poststroke decoction. ScientificWorldJournal. 2013; 2013:896873.
Article11. Kim HT, Park EJ. Change of major functional components of Gastrodia elata Blume with cultivation conditions and harvest times. Korean J Med Crop Sci. 2013; 21:282–288.
Article12. Kim MH, Kim JG, Choi JH. Antioxidant activity and changes in major functional components of fermented Gastrodia elata Blume. Korean J Food Nutr. 2014; 27:684–691.
Article13. Kim HJ, Lee JH, Shin MK, Hyun Leem K, Kim YJ, Lee MH. Inhibitory effect of Gastrodia elata extract on melanogenesis in HM3KO melanoma cells. J Cosmet Sci. 2013; 64:89–98.14. Kim KT, Kim JG, Park SH, Lee JH, Lee SH, Kim KH, Park SN. Anti-melanogenesis effect of phenolic compounds isolated from Gastrodia elata. J Soc Cosmet Sci Korea. 2004; 30:33–38.15. Chen T, Li C, Zhu T, Wu D, Xu W, Han H. Ffects of gastrodin on formation of B16 melanoma cell melanogenesis. J Anhui Agric Sci. 2013; 5280–5282.16. Liu SH, Pan IH, Chu IM. Inhibitory effect of p-hydroxybenzyl alcohol on tyrosinase activity and melanogenesis. Biol Pharm Bull. 2007; 30:1135–1139.
Article17. Wang Y, Lin S, Chen M, Jiang B, Guo Q, Zhu C, Wang S, Yang Y, Shi J. Chemical constituents from aqueous extract of Gastrodia elata. Zhongguo Zhong Yao Za Zhi. 2012; 37:1775–1781.18. Dubost NJ, Beelman RB, Peterson D, Royse DJ. Identification and quantification of ergothioneine in cultivated mushrooms by liquid chromatography-mass spectroscopy. Int J Med Mushrooms. 2006; 8:215–222.
Article19. Cheah IK, Halliwell B. Ergothioneine; antioxidant potential, physiological function and role in disease. Biochim Biophys Acta. 2012; 1822:784–793.
Article20. Chen Y, Tang YM, Yu SL, Han YW, Kou JP, Liu BL, Yu BY. Advances in the pharmacological activities and mechanisms of diosgenin. Chin J Nat Med. 2015; 13:578–587.
Article21. Cronin H, Draelos ZD. Top 10 botanical ingredients in 2010 anti-aging creams. J Cosmet Dermatol. 2010; 9:218–225.22. Shin CS, Park CK, Lee JW, Lee JG, Jang CK, Kim YK. Analysis of the components with freeze drying and steam drying of Gastrodia elata Blume. J Korean Soc Food Sci Nutr. 1999; 28:1058–1063.23. Kim HJ, Chung SK, Moon KD. Chemical components of Gastrodia elata Blume powder. Korean J Postharvest Sci Technol. 2000; 7:278–284.24. Paik YS, Song JK, Yoon CH, Jung KS, Yoon HS. Anti-platelet and anti-thrombotic effects of Gastrodia elata. Korean J Pharmacogn. 1995; 26:385–389.25. Briganti S, Camera E, Picardo M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003; 16:101–110.
Article26. Lee KT, Kim BJ, Kim JH, Heo MY, Kim HP. Biological screening of 100 plant extracts for cosmetic use (I): inhibitory activities of tyrosinase and DOPA auto-oxidation. Int J Cosmet Sci. 1997; 19:291–298.
Article27. Kim CH, Ha BJ, Koo CS, Chang DI. Development of bioconverted skin-whitening cosmetics from Gastrodia elata. Theor Appl Chem Eng. 2009; 15:2512.28. Kim KT, Kim JK, Park SH, Lee JH, Lee SH, Kim KH, Park SN. Anti-melanogenesis effect of phenolic compounds isolated from Gastrodia elata. J Soc Cosmet Sci Korea. 2004; 30:33–38.29. Wu SY, Wang HM, Wen YS, Liu W, Li PH, Chiu CC, Chen PC, Huang CY, Sheu JH, Wen ZH. 4-(Phenylsulfanyl)butan-2-one suppresses melanin synthesis and melanosome maturation in vitro and in vivo. Int J Mol Sci. 2015; 16:20240–20257.
Article30. Pei CJ, Lee J, Si YX, Oh S, Xu WA, Yin SJ, Qian GY, Han HY. Inhibition of tyrosinase by gastrodin: an integrated kinetic-computational simulation analysis. Process Biochem. 2013; 48:162–168.
Article31. Fenoll LG, Rodríguez-López JN, García-Sevilla F, García-Ruiz PA, Varón R, García-Cánovas F, Tudela J. Analysis and interpretation of the action mechanism of mushroom tyrosinase on monophenols and diphenols generating highly unstable o-quinones. Biochim Biophys Acta. 2001; 1548:1–22.
Article32. Bagheri-Kalmarzi M, Sajedi RH, Asadollahi E, Mahmoodi NO, Haji-Hosseini R. Effect of vanillin and its acid and alcohol derivatives on the diphenolase activity of mushroom tyrosinase. Mol Biol Res Commun. 2012; 1:74–82.33. Liao WC, Wu WH, Tsai PC, Wang HF, Liu YH, Chan CF. Kinetics of ergothioneine inhibition of mushroom tyrosinase. Appl Biochem Biotechnol. 2012; 166:259–267.
Article34. Lee J, Jung K, Kim YS, Park D. Diosgenin inhibits melanogenesis through the activation of phosphatidylinositol-3-kinase pathway (PI3K) signaling. Life Sci. 2007; 81:249–254.
Article35. Tada Y, Kanda N, Haratake A, Tobiishi M, Uchiwa H, Watanabe S. Novel effects of diosgenin on skin aging. Steroids. 2009; 74:504–511.
Article36. Song E, Chung H, Shim E, Jeong JK, Han BK, Choi HJ, Hwang J. Gastrodia elata Blume extract modulates antioxidant activity and Ultraviolet A-irradiated skin aging in human dermal fibroblast cells. J Med Food. 2016; 19:1057–1064.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Baicalein Inhibits αα-Melanocyte-stimulating Hormonestimulated Melanogenesis via p38 Mitogen-activated Protein Kinase Pathway in B16F10 Mouse Melanoma Cells
- Superoxide Dismutase 1 Inhibits Alpha-Melanocyte Stimulating Hormone and Ultraviolet B-Induced Melanogenesis in Murine Skin
- Gastrodia elata Blume Attenuates 2, 4-Dinitrochlorobenzene-induced Atopic Dermatitis-like Skin Lesions in Balb/c Mice and SD Rats
- Seed Germination of Gastrodia elata Using Symbiotic Fungi, Mycena osmundicola
- Vitis amurensis Ruprecht root inhibited alpha-melanocyte stimulating hormone-induced melanogenesis in B16F10 cells