Korean J Hematol.
2005 Jun;40(2):93-100. 10.5045/kjh.2005.40.2.93.
Arsenic Trioxide Induces Erythroid Differentiation and Apoptosis of K562 Human Leukemia Cells through the Down-Regulation of Bcl-2
- Affiliations
-
- 1Division of Hematology-Oncology and Institute for Clinical Molecular Biology Research, SoonChunHyang University College of Medicine, Seoul, Korea. jhwon@hosp.sch.ac.kr
- KMID: 2252343
- DOI: http://doi.org/10.5045/kjh.2005.40.2.93
Abstract
- BACKGROUND
Arsenic trioxide (As2O3) has been identified as an effective drug for the treatment of acute promyelocytic leukemia (APL). However, the role of As2O3 during the erythroid differentiation of human leukemic cells remains unknown. In this study, we investigated the in vitro effects of As2O3 on the erythroid differentiation of the K562 cell line and also on the expression and regulation of the apoptotic modulators of this process.
METHODS
The K562 cells were cultured in the presence of 0.1, 0.5 and 1.0micrometer As2O3, or they were cultured in the presence of 1.0 and 10micrometer all trans retinoic acid (ATRA). The expression of glycophorin A before and after treatment with As2O3 or with ATRA in the K562 cells was assessed by flow cytometry and western blotting. The expressions of Bcl-2 and caspase-3 were determined by western blotting.
RESULTS
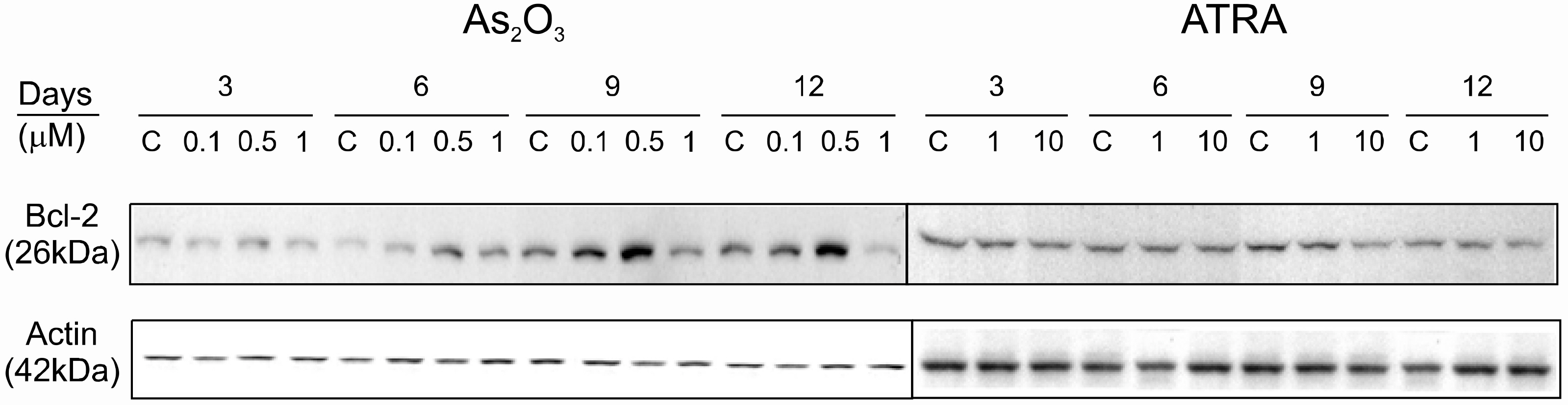
The viability of the K562 cells was not decreased after treating with 0.1 and 0.5micrometer of As2O3, but the viability was significantly reduced at a dose of 1.0micrometer Caspase 3 activation was not observed at 0.1 and 0.5micrometer of As2O3 until 12 days, but Caspase 3 was activated by 1.0micrometer of As2O3 from day 3. The expression of glycophorin A was increased in dose dependent manner by As2O3 treatment, but this was not changed in the ATRA treated K562 cells. The expression of Bcl-2 was increased by 0.1 and 0.5micrometer of As2O3, but it was abruptly reduced by 1.0micrometer of As2O3.
CONCLUSION
These results suggest that As2O3 induces the erythroid differentiation of K562 cells and that 1.0micrometer of As2O3 induces apoptosis through the down-regulation of Bcl-2.
Keyword
MeSH Terms
Figure
Reference
-
1. Cai X, Shen YL, Zhu Q, et al. Arsenic trioxide-induced apoptosis and differentiation are associated respectively with mitochondrial transmembrane potential collapse and retinoic acid signaling pathways in acute promyelocytic leukemia. Leukemia. 2000; 14:262–70.
Article2. Chen GQ, Zhu J, Shi XG, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996; 88:1052–61.3. Chen GQ, Shi XG, Tang W, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyel-ocytoc leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997; 89:3345–53.4. de The H, Chomienne C, Lanotte D, Degos L, Dejean A. The t(15;17) translocation of acute promyelocytic leukemia fuses the retinoic acid receptor alpha gene to a novel transcribed locus. Nature. 1990; 347:558–61.5. Kakizuka A, Miller WH Jr, Umesono K, et al. Chromosomal translocation t(15;17) in acute promyelocytic leukemia fuses RAR alpha with a novel putative transcription factor, PML. Cell. 1991; 66:663–74.6. Rousselot P, Labaume S, Marolleau JP, et al. Arsenic trioxide and melarsoprol induce apoptosis in plasma cell lines and in plasma cells from myeloma patients. Cancer Res. 1999; 59:1041–8.7. Zhang W, Ohnishi K, Shigeno K, et al. The induction of apoptosis and cell cycle arrest by arsenic trioxide in lymphoid neoplasms. Leukemia. 1998; 12:1383–91.
Article8. Walter R, Schoedon G, Bachli E, et al. Establishment and characterization of an arsenic-sensitive monoblastic leukemia cell line (SigM5). Brit J of Haematol. 2000; 109:396–404.9. Rojewski MT, Baldus C, Knauf W, Thiel E, Schre-zenmeier H. Dual effects of arsenic trioxide (As2O3) on non-acute promyelocytic leukaemia myeloid cell lines: induction of apoptosis and inhibition of proliferation. Br J Haematol. 2002; 116:555–63.10. Chen F, Lu Y, Zhang Z, et al. Opposite effect of NF-kappa and c-Jun-N-terminal kinase on p53-independent GADD45 induction by arsenite. J Bio Chem. 2001; 276:11414–9.11. Zhang TC, Cao EH, Li JF, Ma W, Qin JF. Induction of apoptosis and inhibition of human gastric cancer MGC-803 cell growth by arsenic trioxide. Eur J Cancer. 1999; 35:1258–63.
Article12. Seol JG, Park WH, Kim ES, et al. Potential role of caspase-3 and -9 in arsenic trioxide-mediated apoptosis in PCI-1 head and neck cancer cells. Int J Oncol. 2001; 18:249–55.
Article13. Kitamura K, Minami Y, Yamamoto K, et al. Involvement of CD95-independent caspase 8 activation in arsenic trioxide-induced apoptosis. Leukemia. 2000; 14:1743–50.
Article14. Perkins C, Kim CN, Fang G, Bhalla KN. Arsenic induces apoptosis of multidrug-resistant human myeloid leukemia cells that express Bcr-Abl or overexpress MDR, MRP, Bcl-2, or Bcl-x(L). Blood. 2000; 95:1014–22.
Article15. Mahieux R, Pise-Masison C, Gessain A, et al. Arsenic trioxide induces apoptosis in human T-cell leukemia virus type 1- and type 2-infected cells by a caspase-3-dependent mechanism involving Bcl-2 cleavage. Blood. 2001; 98:3762–9.
Article16. Jiang XH, Wong BC, Yuen ST, et al. Arsenic trioxide induces apoptosis in human gastric cancer cells through upregulation of p53 and activation of caspase-3. Int J Cancer. 2001; 91:173–9.
Article17. Woo SH, Park IC, Park MJ, et al. Arsenic trioxide induces apoptosis through a reactive oxygen species- dependent pathway and loss of mitochondrial membrane potential in HeLa cells. Int J Oncol. 2002; 21:57–63.18. Benito A, Silva M, Grillot D, Nunez G, Fernandez-Luna JL. Apoptosis induced by erythroid differentiation of human leukemic cell lines is inhibited by Bcl-XL. Blood. 1996; 87:3837–48.19. Benito A, Grillot D, Nunez G, Fernandez-Luna JL. Regulation and function of Bcl-2 during differentiation-induced cell death in HL-60 promyelocytic cells. Am J Pathol. 1995; 146:481–90.20. Soignet SL, Maslak P, Wang ZG, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998; 339:1341–8.
Article21. Soignet SL, Frankel SR, Douer D, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001; 19:3852–60.
Article22. Miller WH Jr, Schipper HM, Lee JS, Singer J, Waxman S. Mechanisms of action of arsenic trioxide. Cancer Res. 2002; 62:3893–903.23. Gianni M, Kohen MHM, Chelbi-Alix MK, et al. Combined arsenic and retinoic acid treatment enhances differentiation and apoptosis in arsenic-resistant NB4 cells. Blood. 1998; 91:4300–10.24. Naumovki L, Cleary ML. Bcl-2 inhibits apoptosis associated with terminal differentiation of HL-60 myeloid leukemic cells. Blood. 1994; 83:2261–7.25. Park JR, Robertson K, Hickstein DD, Tsai S, Hocken berry DM, Collins SJ. Dysregulated Bcl-2 expression inhibits apoptosis but not differentiation of retinoic acid-induced HL-60 granulocytes. Blood. 1994; 84:440–5.
Article26. Zhu J, Okumura H, Ohtake S, Nakamura S, Nakao S. The molecular mechanism of arsenic trioxide-induced apoptosis and oncosis in leukemia/lymphoma cell lines. Acta Hematol. 2003; 110:1–10.
Article27. Zhang Y, Shen WL. Bcl-2 antisense oligodeoxynu-cleotide increases the sensitivity of leukemic cells to arsenic trioxide. Cell Biol Int. 2003; 27:953–8.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Anticancer Effect of Arsenic Trioxide in Acute Promyelocytic Leukemia
- Inducing Apoptosis of NCI-H157 Human Lung Carcinoma Cells via Activation of Caspase Cascade by Combination Treatment with Arsenic Trioxide and Sulindac
- A Case of Relapsed Acute Promyleocytic Leukemia Induced Remission with Arsenic Trioxide(As2O3)
- Arsenic Trioxide Induces Apoptosis of HL-60 Cells via Activation of Intrinsic Caspase Protease with Mitochondrial Dysfunction
- Combination Treatment with Arsenic Trioxide and Sulindac Induces Apoptosis of NCI-H157 Human Lung Carcinoma Cells via ROS Generation with Mitochondrial Dysfunction