J Lung Cancer.
2006 Dec;5(2):92-95. 10.6058/jlc.2006.5.2.92.
Mutational Analysis of PUMA Gene in Non-small Cell Lung Cancers
- Affiliations
-
- 1Department of Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea. suhulee@catholic.ac.kr
- KMID: 2200168
- DOI: http://doi.org/10.6058/jlc.2006.5.2.92
Abstract
-
PURPOSE: It has become clear that, together with proliferation, deregulation of apoptosis plays a pivotal role in tumorigenesis, and the somatic mutations of apoptosis-related genes have been reported in human cancers. PUMA, a pro- apoptotic member of Bcl-2 family, mediates p53-deependent and -independent apoptosis. The aim of this study was to explore whether alteration of PUMA protein expression is a characteristic of human lung cancers.
MATERIALS AND METHODS
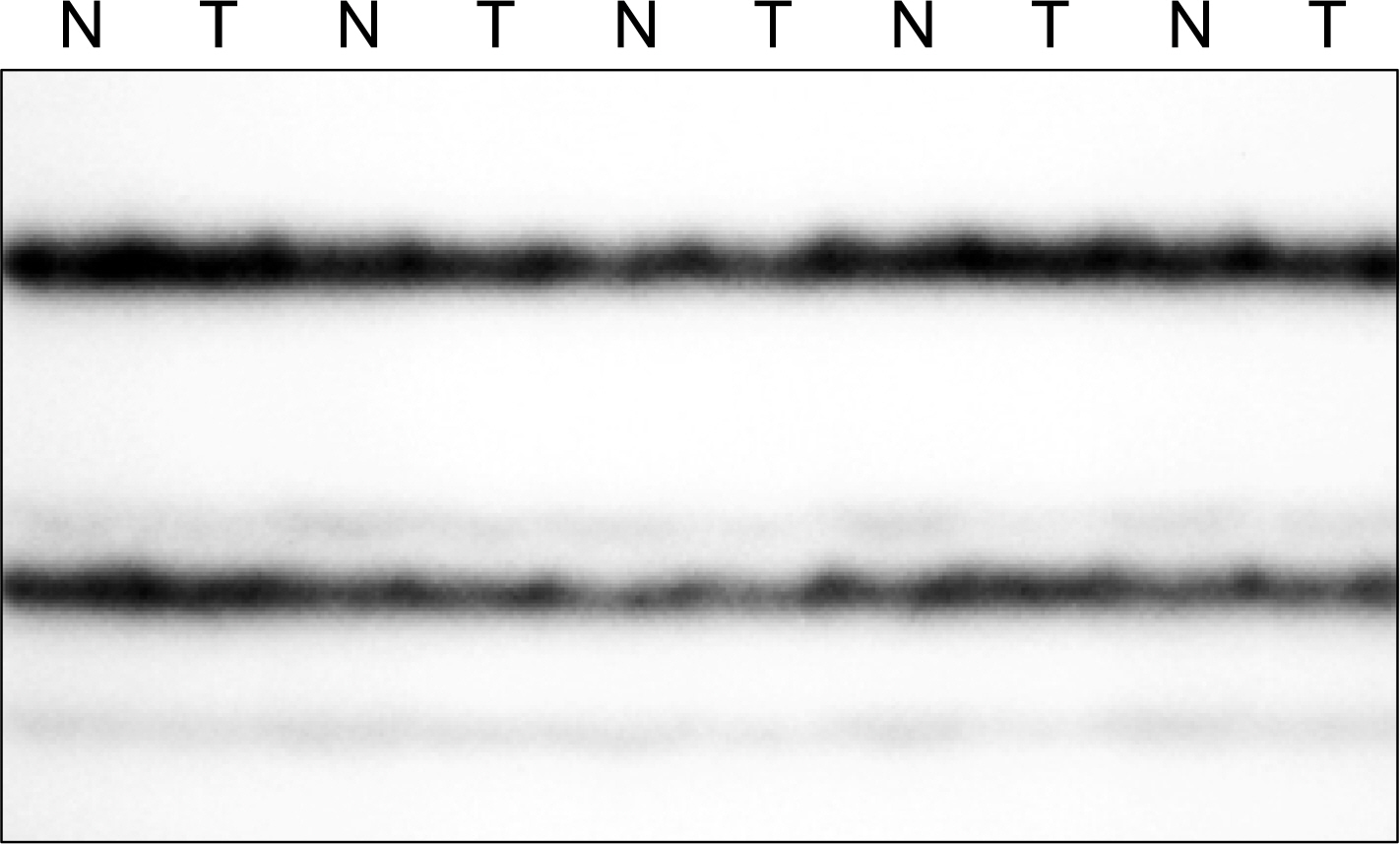
To explore the possibility that the genetic alterations of PUMA might be involved in the development of human cancers, we analyzed the entire coding region and all splice sites of human PUMA gene in 100 human non-small cell lung cancers (NSCLCs) by polymerase chain reaction (PCR)-based single-strand conformation polymorphism (SSCP).
RESULTS
The PCR-SSCP analysis detected no mutation in the entire coding regions and all splice sites of human PUMA gene in the 100 NSCLCs.
CONCLUSION
The data presented here suggested that PUMA gene mutation may not contribute to the pathogenesis of human NSCLCs.
Keyword
MeSH Terms
Figure
Reference
-
1.Reed JC. Mechanisms of apoptosis. Am J Pathol. 2000. 39:1415–1430.
Article2.Nagata S. Apoptosis by death factor. Cell. 1997. 88:355–365.
Article3.Hanahan D., Weinberg RA. The hallmarks of cancer. Cell. 2000. 100:57–70.
Article4.Datta SR., Katsov A., Hu L, et al. 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell. 2000. 6:41–51.
Article5.O'Connor L., Strasser A., O'Reilly LA, et al. Bim: a novel member of the Bcl-2 family that promotes apoptosis. EMBO J. 1998. 17:384–395.6.Inohara N., Ding L., Chen S., Nunez G. Harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins Bcl-2 and Bcl-X (L). EMBO J. 1997. 16:1686–1694.7.Guo B., Godzik A., Reed JC. Bcl-G, a novel pro-apoptotic member of the Bcl-2 family. J Biol Chem. 2001. 276:2780–2785.
Article8.Oda E., Ohki R., Murasawa H, et al. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000. 288:1053–1058.
Article9.Nakano K., Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001. 7:683–694.
Article10.Chang J., Clark GM., Allred DC., Mohsin S., Chamness G., El ledge RM. Survival of patients with metastatic breast carcinoma: importance of prognostic markers of the primary tumor. Cancer. 2003. 97:545–553.11.Krajewska M., Zapata JM., Meinhold-Heerlein I, et al. Expression of Bcl-2 family member Bid in normal and malignant tissues. Neoplasia. 2002. 4:129–140.
Article12.McDonnell TJ., Deane N., Platt FM, et al. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989. 57:79–88.
Article13.Lee JH., Soung YH., Lee JW, et al. Inactivating mutation of the pro-apoptotic gene BID in gastric cancer. J Pathol. 2004. 202:439–445.14.Kondo S., Shinomura Y., Miyazaki Y, et al. Mutations of the bak gene in human gastric and colorectal cancers. Cancer Res. 2000. 60:4328–4330.15.Rampino N., Yamamoto H., Ionov Y, et al. Somatic frameshift mutations in the BAX gene in colon cancers of the microsatellite mutator phenotype. Science. 1997. 275:967–969.16.Lee JW., Soung YH., Kim SY, et al. Inactivating mutations of proapoptotic Bad gene in human colon cancers. Carcinogenesis. 2004. 25:1371–1376.
Article17.Lee SH., Shin MS., Park WS, et al. Alterations of Fas (Apo-1/CD95) gene in non-small cell lung cancer. Oncogene. 1999. 18:3754–3760.
Article18.Lee SH., Shin MS., Kim HS, et al. Alterations of the DR5/TRAIL receptor 2 gene in non-small cell lung cancers. Cancer Res. 1999. 59:5683–5686.19.Soung YH: Lee JW: Kim SY, et al. Caspase-8 gene is frequently inactivated by the frameshift somatic mutation 1225_1226delTG in hepatocellular carcinomas. Oncogene. 2005. 24:141–147.
Article20.Kim HS., Lee JW., Soung YH, et al. Inactivating mutations of caspase-8 gene in colorectal carcinomas. Gastroenterology. 2003. 125:708–715.
Article21.Soung YH., Lee JW., Kim SY, et al. CASPASE-8 gene is inactivated by somatic mutations in gastric carcinomas. Cancer Res. 2005. 65:815–821.22.Soung YH., Lee JW., Kim HS, et al. Inactivating mutations of CASPASE-7 gene in human cancers. Oncogene. 2003. 22:8048–8052.
Article23.Soung YH., Lee JW., Kim HS, et al. Somatic mutations of CASP3 gene in human cancers. Hum Genet. 2004. 115:112–115.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mutational Analysis of Pro-apoptotic BNIP3 Gene in Non- Small Cell Lung Cancers
- Mutational and Expressional Analysis of ATG5 Gene in Non-Small Cell Lung Cancers
- Mutational and Expressional Analysis of DOK2 Gene in Non-small Cell Lung Cancers
- Pro-apoptotic Cytochrome c Gene Mutation is Rare in Non-small Cell Lung Cancers
- Mutational Analysis of Pro-apoptotic BAD Gene in Nonsmall Cell Lung Cancer