J Korean Assoc Oral Maxillofac Surg.
2012 Apr;38(2):101-109. 10.5125/jkaoms.2012.38.2.101.
Impact of methylation of the p16INK4a gene on the prognosis ofhead and neck squamous cell carcinoma patients
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, School of Dentistry, Pusan National University, Yangsan, Korea. ydkimdds@pusan.ac.kr
- KMID: 2189706
- DOI: http://doi.org/10.5125/jkaoms.2012.38.2.101
Abstract
OBJECTIVES
The inactivation of the tumor suppressor gene p16INK4a plays an important role in the development of malignant tumors, including oral squamous cell carcinoma. The p16 gene is involved in the p16/cyclin-dependent kinase/retinoblastoma (Rb) gene pathway of cell cycle control. The p16 protein is considered a negative regulator of this pathway. The p16 gene encodes an inhibitor of cyclin-dependent kinases 4 and 6 which regulate the phosphorylation of the retinoblastoma gene and G1 to S phase transition in the cell cycle. However, the p16 gene can lose its functionality through point mutations, loss of heterozygosity or methylation of its promoter region.
MATERIALS AND METHODS
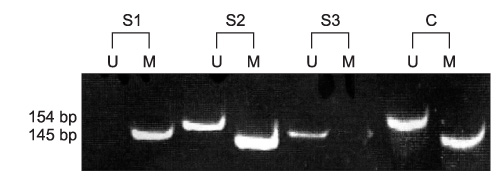
In this study, the authors analyzed the correlation between various clinicopathological findings-patient age, gender and smoking, disease recurrence, tumor size, stage, and differentiation- and p16 protein expression or p16 promoter hypermethylation in 59 cases of head and neck squamous cell carcinoma.
RESULTS
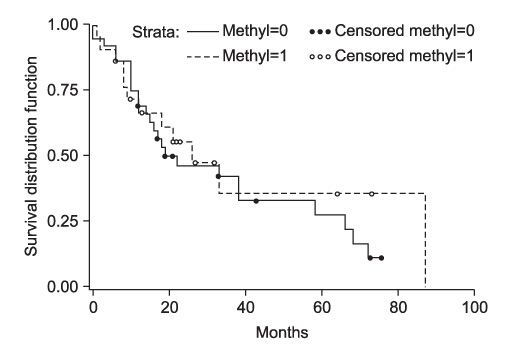
The results revealed p16 protein expression and p16 promoter hypermethylation in 28 cases (47.5%) and 21 cases (35.6%), respectively, of head and neck squamous cell carcinoma. However, neither p16 protein expression nor p16 promoter hypermethylation had any statistical influence on clinicopathological findings or survival rate.
CONCLUSION
This data, and a review of the literature, suggest that p16 promoter hypermethylation cannot yet be used as an independent prognostic factor influencing carcinogenesis, but must be considered as an important factor along with other genetic alterations affecting the pRb pathway.
Keyword
MeSH Terms
Figure
Reference
-
1. Feinberg AP, Tycko B. The history of cancer epigenetics. Nat Rev Cancer. 2004. 4:143–153.
Article2. Santos KF, Mazzola TN, Carvalho HF. The prima donna of epigenetics: the regulation of gene expression by DNA methylation. Braz J Med Biol Res. 2005. 38:1531–1541.
Article3. Okami K, Sakai A, Onuki J, Hamano T, Iida M, Takahashi M. Promoter hypermethylation of tumor-associated genes in head and neck cancer. Nihon Jibiinkoka Gakkai Kaiho. 2005. 108:207–213.
Article4. Dei Tos AP, Maestro R, Doglioni C, Piccinin S, Libera DD, Boiocchi M, et al. Tumor suppressor genes and related molecules in leiomyosarcoma. Am J Pathol. 1996. 148:1037–1045.5. Koscielny S, V Eggeling F, Dahse R. Investigations to the influence of tumor supressor gene p16 inactivation on the prognosis of head and neck squamous cell carcinoma. Laryngorhinootologie. 2004. 83:374–380.
Article6. O'Sullivan B, Shah J. New TNM staging criteria for head and neck tumors. Semin Surg Oncol. 2003. 21:30–42.7. Kratzke RA, Greatens TM, Rubins JB, Maddaus MA, Niewoehner DE, Niehans GA, et al. Rb and p16INK4a expression in resected non-small cell lung tumors. Cancer Res. 1996. 56:3415–3420.8. Geradts J, Kratzke RA, Niehans GA, Lincoln CE. Immunohistochemical detection of the cyclin-dependent kinase inhibitor 2/multiple tumor suppressor gene 1 (CDKN2/MTS1) product p16INK4A in archival human solid tumors: correlation with retinoblastoma protein expression. Cancer Res. 1995. 55:6006–6011.9. Burri N, Shaw P, Bouzourene H, Sordat I, Sordat B, Gillet M, et al. Methylation silencing and mutations of the p14ARF and p16INK4a genes in colon cancer. Lab Invest. 2001. 81:217–229.
Article10. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958. 53:457–481.
Article11. Bartkova J, Lukas J, Bartek J. Aberrations of the G1- and G1/S-regulating genes in human cancer. Prog Cell Cycle Res. 1997. 3:211–220.
Article12. Ai L, Stephenson KK, Ling W, Zuo C, Mukunyadzi P, Suen JY, et al. The p16 (CDKN2a/INK4a) tumor-suppressor gene in head and neck squamous cell carcinoma: a promoter methylation and protein expression study in 100 cases. Mod Pathol. 2003. 16:944–950.
Article13. Cordon-Cardo C. Mutations of cell cycle regulators. Biological and clinical implications for human neoplasia. Am J Pathol. 1995. 147:545–560.14. Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993. 366:704–707.
Article15. Sherr CJ. Cancer cell cycles. Science. 1996. 274:1672–1677.
Article16. Weber A, Wittekind C, Tannapfel A. Genetic and epigenetic alterations of 9p21 gene products in benign and malignant tumors of the head and neck. Pathol Res Pract. 2003. 199:391–397.
Article17. Reed JA, Loganzo F Jr, Shea CR, Walker GJ, Flores JF, Glendening JM, et al. Loss of expression of the p16/cyclin-dependent kinase inhibitor 2 tumor suppressor gene in melanocytic lesions correlates with invasive stage of tumor progression. Cancer Res. 1995. 55:2713–2718.18. Bartsch D, Shevlin DW, Callery MP, Norton JA, Wells SA Jr, Goodfellow PJ. Reduced survival in patients with ductal pancreatic adenocarcinoma associated with CDKN2 mutation. J Natl Cancer Inst. 1996. 88:680–682.
Article19. Takeuchi H, Ozawa S, Ando N, Shih CH, Koyanagi K, Ueda M, et al. Altered p16/MTS1/CDKN2 and cyclin D1/PRAD-1 gene expression is associated with the prognosis of squamous cell carcinoma of the esophagus. Clin Cancer Res. 1997. 3:2229–2236.20. Daniel FI, Cherubini K, Yurgel LS, de Figueiredo MA, Salum FG. The role of epigenetic transcription repression and DNA methyltransferases in cancer. Cancer. 2011. 117:677–687.
Article21. Liu HW, Hu BQ, Cao CF. The p16 methylation in oral leukoplakia and oral squamous cell carcinoma. Zhonghua Kou Qiang Yi Xue Za Zhi. 2005. 40:94–97.22. López M, Aguirre JM, Cuevas N, Anzola M, Videgain J, Aguirre-gaviria J, et al. Gene promoter hypermethylation in oral rinses of leukoplakia patients--a diagnostic and/or prognostic tool? Eur J Cancer. 2003. 39:2306–2309.
Article23. Nakahara Y, Shintani S, Mihara M, Matsumura T, Hamakawa H. High frequency methylation of p16INK4A gene during 4-nitroquinoline 1-oxide-induced rat tongue carcinogenesis. Oncol Rep. 2004. 12:101–106.
Article24. Viswanathan M, Tsuchida N, Shanmugam G. Promoter hypermethylation profile of tumor-associated genes p16, p15, hMLH1, MGMT and E-cadherin in oral squamous cell carcinoma. Int J Cancer. 2003. 105:41–46.
Article25. Nowak MA, Michor F, Komarova NL, Iwasa Y. Evolutionary dynamics of tumor suppressor gene inactivation. Proc Natl Acad Sci U S A. 2004. 101:10635–10638.
Article26. Huang MJ, Yeh KT, Shih HC, Wang YF, Lin TH, Chang JY, et al. The correlation between CpG methylation and protein expression of P16 in oral squamous cell carcinomas. Int J Mol Med. 2002. 10:551–554.27. Goldenberg D, Harden S, Masayesva BG, Ha P, Benoit N, Westra WH, et al. Intraoperative molecular margin analysis in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004. 130:39–44.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Alterations of p16INK4A Gene in Korean Squamous Cell Carcinoma and Basal Cell carcinoma of the Skin
- The Overexpression of Histone Deacetylase 1 and Its Relationship with p16INK4a Gene Hypermethylation in Pulmonary Squamous Cell Carcinoma and Adenocarcinoma
- Role of DCC(Deleted in Colorectal Cancer) Gene in Oral Squamous Cell Carcinoma
- Promoter Methylation of CDKN2A, RARbeta, and RASSF1A in Non-Small Cell Lung Carcinoma: Quantitative Evaluation Using Pyrosequencing
- Detection of Aberrant p16INK4A Methylation in Sera of Patients with Liver Cirrhosis and Hepatocellular Carcinoma