J Korean Med Assoc.
2006 Aug;49(8):717-730. 10.5124/jkma.2006.49.8.717.
Pathogenesis of Alzheimer's Dementia
- Affiliations
-
- 1Department of Pharmacology, Seoul National University College of Medicine, Korea. yhsuh@snu.ac.kr
- KMID: 2184701
- DOI: http://doi.org/10.5124/jkma.2006.49.8.717
Abstract
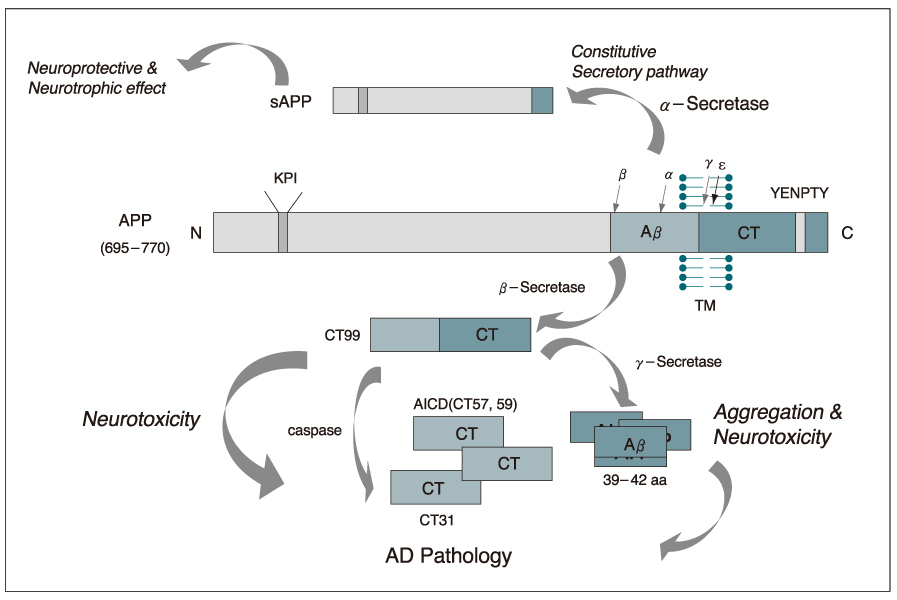
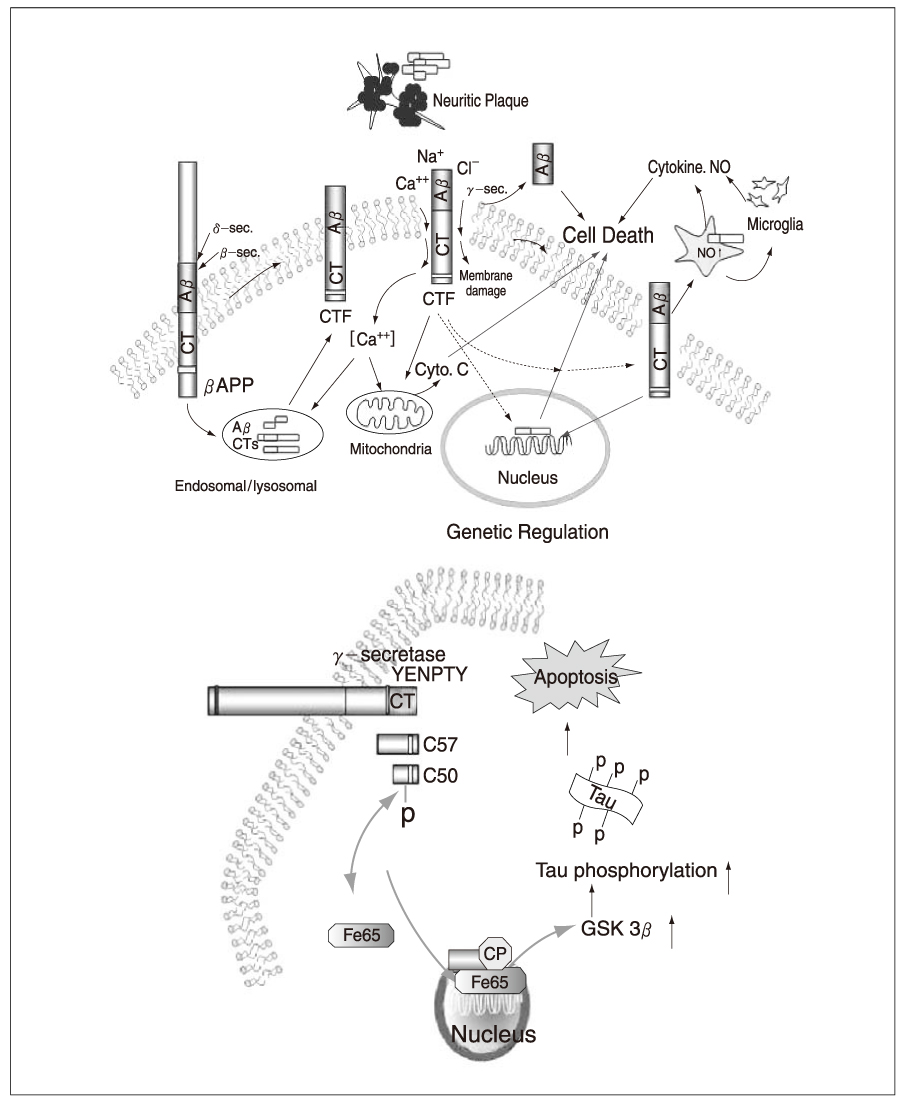
- Alzhelmer's disease (AD) is the most common cause of dementia that arises on a neuropathological background of amyloid plaques containing betaamylold (Abeta) derived from amyloid precursor protein (APP) and tau-rich neurofibrillary tangles. To date, the cause and progression of familial or sporadic AD have not been fully elucidated. About 10% of all cases of AD occur as autosomal dominant inherited forms of early-onset AD, which are caused by mutations in the genes encoding APP, presenilin-1 and presenilin-2. Proteolytic processing of APP by beta-gamma-secretase and caspase generates Abetaand carboxyl-terminal fragments of APP (APP-CTFs), which have been implicated in the pathogenesis of AD. The presenilins function as one of the gamma-secretases. Abetawhich is the main component of the amyloid plaques found, is known to exert neurotoxicity by accumulating free radicals, disturbing calcium homeostasis, evoking inflammatory response and activating signaling pathways. The CTFs have been found in AD patients' brain and reported to exhibit much greater neurotoxicity than Abeta. Furthermore CTFs are known to impair calcium homeostasis and learning and memory, triggering a strong inflammatory reaction through MAPKs- and NF-kappaB-dependent astrocytosis and iNOS induction. Recently, it was reported that CTF translocated into the nucleus and in turn, affected transcription of genes including glycogen synthase kinase-3beta which results in the induction of tau-rich neurofibrillary tangles and subsequently cell death. One of the hallmarks of AD, neurofibrillary tangles (NFT), is formed by insoluble intracellular polymers of hyperphosphorylated tau that is believed to cause apoptosis by disrupting cytoskeletal and axonal transport. This review covers the processing of APP, toxic mechanisms of Abetaand CTFs of APP, presenilin and also tau in relation to the pathogenesis of AD.
Keyword
MeSH Terms
Figure
Reference
-
1. Alzheimer A. Uber eine eigenartige Erkrankung der Hirnrinde. Allg Zeitschr Psychiatr Psychiatr-Gerichtl Med. 1907. 146–148.2. Suh YH, Checler F. Amyloid Precursor Protein, Presenilins, and α-synuclein: Molecular Pathogenesis and Pharmacological Applications in Alzheimer's disease. Pharmacol Rev. 2002. 54:469–525.
Article3. Suh YH. An etiological role of amyloidogenic carboxyl-terminal fragments of the beta-amyloid precursor protein in Alzheimer's disease. J Neurochem. 1997. 68:1781–1791.
Article4. Selkoe DJ. Alzheimer's disease: genes, proteins, and therapy. Physiol Rev. 2001. 81:741–766.
Article5. Sato T, Dohmae N, Qi Y, Kakuda N, Misonou H, Mitsumori R, et al. Potential link between amyloid beta-protein 42 and Cterminal fragment gamma 49-99 of beta-amyloid precursor protein. J Biol Chem. 2003. 278:24294–24301.
Article6. Lu DC, Rabizadeh S, Chandra S, Shayya RF, Ellerby LM, Bredesen DE, et al. A second cytotoxic proteolytic peptide derived from amyloid beta-protein precursor. Nat Med. 2000. 6:397–404.
Article7. Su JH, Zhao M, Anderson AJ, Srinivasan A, Cotman CW. Activated caspase-3 expression in Alzheimer's and aged control brain: correlation with Alzheimer pathology. Brain Res. 2001. 898:350–357.
Article8. Kim HS, Kim EM, Lee JP, Park CH, Kim S, Suh YH, et al. C-terminal fragments of Amyloid Precursor Protein Exert Neurotoxicity by Inducing Glycogen Synthase Kinase-3β, Expression. FASEB J. 2003. 17:1951–1953.
Article9. Chang KA, Kim HS, Ha TY, Ha JW, Shin KY, Suh YH, et al. Phosphorylation of APP at Thr668 regulates the nuclear translocation of AICD and induces neurodegeneration. Molecular and cellular biology. 2006. 26:4327–4338.
Article10. Neve RL, McPhie DL, Chen Y. Alzheimer's disease: a dysfunction of the amyloid precursor protein(1). Brain Res. 2000. 886:54–56.11. Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Selkoe DJ, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002. 416:535–539.
Article12. Vitek MP, Bhattacharya K, Glendening JM, Stopa E, Vlassara H, Cerami A, et al. Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc Natl Acad Sci USA. 1994. 91:4766–4770.
Article13. Lin H, Zhu YJ, Lal R. Amyloid beta protein (1~40) forms calcium-permeable, Zn2+- sensitive channel in reconstituted lipid vesicles. Biochemistry. 1999. 38:11189–11196.
Article14. Zhu YJ, Lin H, Lal R Fresh, nonfibrillar . Amyloid beta protein(1-40) induces rapid cellular degeneration in aged human fibroblasts: evidence for AbetaP-channel-mediated cellular toxicity. FASEB J. 2000. 14:1244–1254.
Article15. Green KN, Peers C. Amyloid beta peptides mediate hypoxic augmentation of Ca(2+) channels. J Neurochem. 2001. 77:953–956.16. Lin H, Bhatia R, Lal R. Amyloid beta protein forms ion channels: implications for Alzheimer's disease pathophysiology. FASEB J. 2001. 15:2433–2444.
Article17. Wu A, Derrico CA, Hatem L, Colvin RA. Alzheimer's amyloid-beta peptide inhibits sodium/calcium exchange measured in rat and human brain plasma membrane vesicles. Neuroscience. 1997. 80:675–684.
Article18. Good TA, Smith DO, Murphy RM. Beta-amyloid peptide blocks the fast-inactivating K+ current in rat hippocampal neurons. Biophys J. 1996. 70:296–304.
Article19. Mark RJ, Hensley K, Butterfield DA, Mattson MP. Amyloid b-peptide impairs ion-motive ATPase activities: evidence for a role in loss of neuronal Ca2+ homeostasis and cell death. J Neurosci. 1995. 15:6239–6249.
Article20. Behl C, Davis JB, Lesley R, Chubert D. Hydrogen Peroxide Mediates Amyloid Protein Toxicity. Cell. 2000. 77:817–827.
Article21. Butterfield DA, Hensley K, Harris M, Mattson MP, Carney J. Beta-amyloid peptide free radical fragments initiate synaptosomal lipoperoxidation in a sequence-specific fashion: implications to Alzheimer's disease. Biochem Biophys Res Commun. 1994. 200:710–715.
Article22. Mattson MP, Lovell MA, Furukawa K, Markesbery WR. Neurotrophic factors attenuate glutamate-induced accumulation of peroxides, elevation of intracellular calcium concentration, and neurotoxicity and increase antioxidant enzyme activities in hippocampal neurons. J Neurochem. 1995. 65:1740–1751.
Article23. Varadarajan S, Yatin S, Aksenova M, Butterfield AD. Alzheimer's amyloid beta peptide-associated free radical oxidative stress and neurotoxicity. Journal of Structural Biology. 2000. 130:184–208.
Article24. Monji A, Utsumi H, Ueda T, Imoto T, Yoshida I, Tashiro N, et al. The relationship between the aggregational state of the amyloid-beta peptides and free radical generation by the peptides. J Neurochem. 2001. 77:1425–1432.
Article25. Michikawa M, Gong JS, Fan QW, Sawamura N, Yanagisawa K. A novel action of alzheimer's amyloid beta-protein (Abeta): oligomeric Abeta promotes lipid release. J Neurosci. 2001. 21:7226–7235.
Article26. Tan J, Town T, Paris D, Mori T, Suo Z, Mullan M, et al. Microglial activation resulting from CD40-CD40L interaction after beta-amyloid stimulation. Science. 1999. 286:2352–2355.
Article27. Lue LF, Rydel R, Brigham EF, Yang LB, Hampel H, Rogers J, et al. Inflammatory repertoire of Alzheimer's disease and nondemented elderly microglia in vitro. Glia. 2001. 35:72–79.
Article28. Rapoport M, Ferreira A. PD98059 prevents neurite degeneration induced by fibrillar beta-amyloid in mature hippocampal neurons. J Neurochem. 2000. 74:125–133.
Article29. Morishima Y, Gotoh Y, Zieg J, Barrett T, Takano H, Greenberg ME, et al. Beta-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J Neurosci. 2001. 2:7551–7560.30. Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH. Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature. 1999. 402:615–622.
Article31. Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH. Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature. 2000. 405:360–364.
Article32. Williamson R, Scales T, Clark BR, Gibb G, Reynolds CH, Anderton BH, et al. Rapid tyrosine phosphorylation of neuronal proteins including tau and focal adhesion kinase in response to amyloid-beta peptide exposure: involvement of Src family protein kinases. J Neurosci. 2002. 22:10–20.
Article33. Ghiso J, Rostagno A, Gardella JE, Liem L, Gorevic PD, Frangione B. A 109-amino-acid C-terminal fragment of Alzheimer's-disease amyloid precursor protein contains a sequence,-RHDS-, that promotes cell adhesion. Biochem J. 1992. 288:1053–1059.
Article34. Gardella JE, Gorgone GA, Candela L, Ghiso J, Castano EM, Frangione B, et al. High-level expression and in vitro mutagenesis of a fibrillogenic 109-amino-acid C-terminal fragment of Alzheimer's-disease amyloid precursor protein. Biochem J. 1993. 294:667–674.
Article35. Li QX, Evin G, Small DH, Multhaup G, Beyreuther K, Masters CL. Proteolytic processing of Alzheimer's disease beta A4 amyloid precursor protein in human platelets. J Biol Chem. 1995. 270:14140–14147.
Article36. Matsumoto A, Fujiwara Y. Abnormal and deficient processing of beta-amyloid precursor protein in familial Alzheimer's disease lymphoblastoid cells. Biochem Biophys Res Commun. 1991. 175:361–365.
Article37. Matsumoto A, Fujiwara Y. Aberrant proteolysis of the beta-amyloid precursor protein in familial Alzheimer's disease lymphoblastoid cells. Eur J Biochem. 1993. 217:21–27.
Article38. Matsumoto A, Matsumoto R. Familial Alzheimer's disease cells abnormally accumulate beta-amyloid-harbouring peptides preferentially in cytosol but not in extracellular fluid. Eur J Biochem. 1994. 225:1055–1062.
Article39. Tokuda T, Tanaka K, Kametani F, Ikeda S, Yanagisawa N. Secretory cleavage of beta-amyloid precursor protein in the cerebral white matter produces amyloidogenic carboxyl-terminal fragments. Neurosci Lett. 1995. 186:149–152.
Article40. Nordstedt C, Gandy SE, Alafuzoff I, Caporaso GL, Iverfeldt K, Grebb JA, et al. Alzheimer beta/A4 amyloid precursor protein in human brain: aging-associated increases in holoprotein and in a proteolytic fragment. Proc Natl Acad Sci USA. 1991. 88:8910–8914.
Article41. Estus S, Golde TE, Kunishita T, Blades D, Lowery D, Eisen M, et al. Potentially amyloidogenic, carboxyl-terminal derivatives of the amyloid protein precursor. Science. 1992. 255:726–728.
Article42. Tamaoka A, Kalaria RN, Lieberburg I, Selkoe DJ. Identification of a stable fragment of the Alzheimer amyloid precursor containing the beta-protein in brain microvessels. Proc Natl Acad Sci USA. 1992. 89:1345–1349.
Article43. Amaratunga A, Fine RE. Generation of amyloidogenic C-terminal fragments during rapid axonal transport in vivo of betaamyloid precursor protein in the optic nerve. J Biol Chem. 1995. 270:17268–17272.
Article44. Nalbantoglu J, Tirado-Santiago G, Lahsaini A, Poirier J, Goncalves O, Verge G, et al. Impaired learning and LTP in mice expressing the carboxy terminus of the Alzheimer amyloid precursor protein. Nature. 1997. 387:500–505.
Article45. Tanzi RE, Bertram L. New frontiers in Alzheimer's disease genetics. Neuron. 2001. 32:181–184.
Article46. Lee JP, Chang KA, Kim HS, Kim SS, Jeong SJ, Suh YH. APP carboxyl-terminal fragment without or with abeta domain equally induces cytotoxicity in differentiated PC12 cells and cortical neurons. J Neurosci Res. 2000. 60:565–570.
Article47. Marcon G, Giaccone G, Canciani B, Cajola L, Rossi G, De Gioia L, et al. A betaPP peptide carboxyl-terminal to Abeta is neurotoxic. Am J Pathol. 1999. 154:1001–1007.48. Song DK, Won MH, Jung JS, Lee JC, Kang TC, Suh HW, et al. Behavioral and neuropathologic changes induced by central injection of carboxyl-terminal fragment of beta-amyloid precursor protein in mice. J Neurochem. 1998. 71:875–878.
Article49. Choi SH, Park CH, Koo JW, Seo JH, Kim HS, Jeong SJ, et al. Memory impairment and cholinergic dysfunction by centrally administered Abeta and carboxyl-terminal fragment of Alzheimer's APP in mice. FASEB J. 2001. 15:1816–1818.
Article50. Lambourne SL, Sellers LA, Bush TG, Choudhury SK, Emson PC, Suh YH, et al. Increased tau phosphorylation on mitogenactivated protein kinase consensus sites and cognitive decline in transgenic models for Alzheimer's disease and FTDP-17: evidence for distinct molecular processes underlying tau abnormalities. Mol Cell Biol. 2005. 25:278–293.
Article51. Kammesheidt A, Boyce FM, Spanoyannis AF, Cummings BJ, Ortegon M, Cotman C, et al. Deposition of beta/A4 immuno-reactivity and neuronal pathology in transgenic mice expressing the carboxyl-terminal fragment of the Alzheimer amyloid precursor in the brain. Proc Natl Acad Sci USA. 1992. 89:10857–10861.
Article52. Ando K, Iijima KI, Elliott JI, Kirino Y, Suzuki T. Phosphorylation-dependent regulation of the interaction of amyloid precursor protein with Fe65 affects the production of beta-amyloid. J Biol Chem. 2001. 276:40353–40361.
Article53. Fraser SP, Suh YH, Djamgoz MBA. Ionic effects of the Alzheimer's disease-amyloid precursor protein and its metabolic fragments. Trends Neurosci. 1997. 20:67–72.
Article54. Fraser SP, Suh YH, Chong YH, Djamgoz MB. Membrane currents induced in Xenopus oocytes by the C-terminal fragment of the beta-amyloid precursor protein. J Neurochem. 1996. 66:2034–2040.
Article55. Kim JH, Rah JC, Fraser SP, Chang KA, Djamgoz MB, Suh YH. Carboxyl-terminal peptide of beta-amyloid precursor protein blocks inositol 1, 4, 5-trisphosphate-sensitive Ca2+ release in Xenopus laevis oocytes. J Biol Chem. 2002. 277:20256–20263.
Article56. Hartell NA, Suh YH. Peptide fragments of beta-amyloid precursor protein: effects on parallel fiber-Purkinje cell synaptic transmission in rat cerebellum. J Neurochem. 2000. 74:1112–1121.57. Kim HS, Lee JH, Suh YH. C-terminal fragment of Alzheimer's amyloid precursor protein inhibits sodium/calcium exchanger activity in SK-N-SH cell. Neuroreport. 1999. 10:113–116.
Article58. Arispe N, Rojas E, Pollard HB. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc Natl Acad Sci USA. 1993. 90:567–571.
Article59. McKeon-O'Malley C, Wells J, Fine R, Ullman MD, Volicer L. PC12 cells transfected with a C-terminal fragment of the amyloid precursor protein (APP C-100), exhibit enhanced sensitivity to the calcium ionophore A23187, and diminished sensitivity to hydrogen peroxide. Brain Res Mol Brain Res. 1999. 72:103–107.60. Kim HS, Park CH, Cha SH, Lee JH, Lee S, Kim Y, et al. Carboxyl-terminal fragment of Alzheimer's APP destabilizes calcium homeostasis and renders neuronal cells vulnerable to excitotoxicity. FASEB J. 2000. 14:1508–1517.
Article61. Cullen WK, Suh YH, Anwyl R, Rowan MJ. Block of LTP in rat hippocampus in vivo by beta-amyloid precursor protein fragments. Neuroreport. 1997. 8:3213–3217.
Article62. Kim JH, Anwyl R, Suh YH, Djamgoz MB, Rowan MJ. Use-dependent effects of amyloidogenic fragments of (beta)-amyloid precursor protein on synaptic plasticity in rat hippocampus in vivo. J Neurosci. 2001. 21:1327–1333.
Article63. Sato M, Kawarabayashi T, Shoji M, Kobayashi T, Tada N, Matsubara E, et al. Neurodegeneration and gliosis in transgenic mice overexpressing a carboxy-terminal fragment of Alzheimer amyloid-beta protein precursor. Dement Geriatr Cogn Disord. 1997. 8:296–307.
Article64. Bach JH, Chae HS, Rah JC, Lee MW, Park CH, Choi SH, et al. C-terminal fragment of amyloid precursor protein induces astrocytosis. J Neurochem. 2001. 78:109–120.
Article65. Rah JC, Kim HS, Kim SS, Bach JH, Kim YS, Park CH, et al. Effects of carboxyl-terminal fragment of Alzheimer's amyloid precursor protein and amyloid beta-peptide on the production of cytokines and nitric oxide in glial cells. FASEB J. 2001. 15:1463–1465.
Article66. Chong YH, Sung JH, Shin SA, Chung JH, Suh YH. Effects of the-amyloid and carboxy-terminal fragment of Alzheimer's amyloid precursor protein on the production of the tumor necrosis factor-and matrix metalloproteinase-9 by human monocytic THP-1. J Biol Chem. 2001. 276:23511–23517.
Article67. DeGiorgio LA, DeGiorgio N, Milner TA, Conti B, Volpe BT. Neurotoxic APP C-terminal and beta-amyloid domains colocalize in the nuclei of substantia nigra pars reticulata neurons undergoing delayed degeneration. Brain Res. 2000. 874:137–146.
Article68. Cao X, Sudhof TC. A transcriptionally [correction of transcriptively] active complex of APP with Fe65 and histone acetyltransferase Tip60. Science. 2001. 293:115–120.
Article69. Kimberly WT, Zheng JB, Guenette SY, Selkoe DJ. The intracellular domain of the beta-amyloid precursor protein is stabilized by Fe65 and translocates to the nucleus in a notch-like manner. J Biol Chem. 2001. 276:40288–40292.
Article70. Gao Y, Pimplikar SW. The gamma-secretase-cleaved Cterminal fragment of amyloid precursor protein mediates signaling to the nucleus. Proc Natl Acad Sci USA. 2001. 98:14979–14984.
Article71. Cupers P, Bentahir M, Craessaerts K, Orlans I, Vanderstichele H, Saftig P, et al. The discrepancy between presenilin subcellular localization and-secretase processing of amyloid precursor protein. J Cell Biol. 2001. 154:731–740.
Article72. Xu Y, Kim HS, Joo Y, Choi Y, Chang KA, Suh YH, et al. Intracellular Domains of Amyloid Precursor-Like Protein 2 Interact with CP2 Transcription Factor in the Nucleus and Induce Glycogen Synthase Kinase-3β, Expression. Cell Death and Differentiation. 2006. doi: 10.1038/sj.cdd.4401928.
Article73. Hardy J. Amyloid, the presenilins and Alzheimer's disease. Trends Neurosci. 1997. 20:154–159.
Article74. Prihar G, Fuldner RA, Perez-Tur J, Lincoln S, Duff K, Adams MD, et al. Structure and alternative splicing of the presenilin-2 gene. Neuroreport. 1996. 7:1680–1684.
Article75. Borchelt DR, Ratovitski T, Van Lare J, Lee MK, Gonzales V, Sisodia SS, et al. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997. 19:939–945.
Article76. Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, Duff K, et al. Accerlated Alzheimer-type phenotype in transgenic micecarrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nature Medicine. 1998. 4:97–100.
Article77. Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Mc-Gowan E, et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001. 293:1487–1491.
Article78. Gotz J, Chen F, van Dorpe J, Nitsch RM. Formation of neurofibrillary tangles in P301l tau transgenic mice induced by Abeta 42 fibrils. Science. 2001. 293:1491–1495.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biomarkers for Alzheimer's Dementia : Focus on Neuroimaging
- Significance of Non-Alzheimer Dementia
- Relationship Between Sleep and Alzheimer’s Dementia
- Differential Diagnosis of Vascular Dementia and Alzheimer's Disease
- The Conceptual History of Dementia and Alzheimer's Disease: Focus on Alzheimer's Disease