J Gynecol Oncol.
2012 Jan;23(1):35-42. 10.3802/jgo.2012.23.1.35.
BCL2 antagonist of cell death kinases, phosphatases, and ovarian cancer sensitivity to cisplatin
- Affiliations
-
- 1Department of Women's Oncology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA. Johnathan.lancaster@moffitt.org
- 2Experimental Therapeutics Program, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
- 3Department of Anatomic Pathology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
- KMID: 2177512
- DOI: http://doi.org/10.3802/jgo.2012.23.1.35
Abstract
OBJECTIVE
The BCL2 family proteins are critical mediators of cellular apoptosis and, as such, have been implicated as determinants of cancer cell chemo-sensitivity. Recently, it has been demonstrated that the phosphorylation status of the BCL2 antagonist of cell death (BAD) protein may influence ovarian cancer (OVCA) cell sensitivity to cisplatin. Here, we sought to evaluate how kinase and phosphatase components of the BAD apoptosis pathway influence OVCA chemo-sensitivity.
METHODS
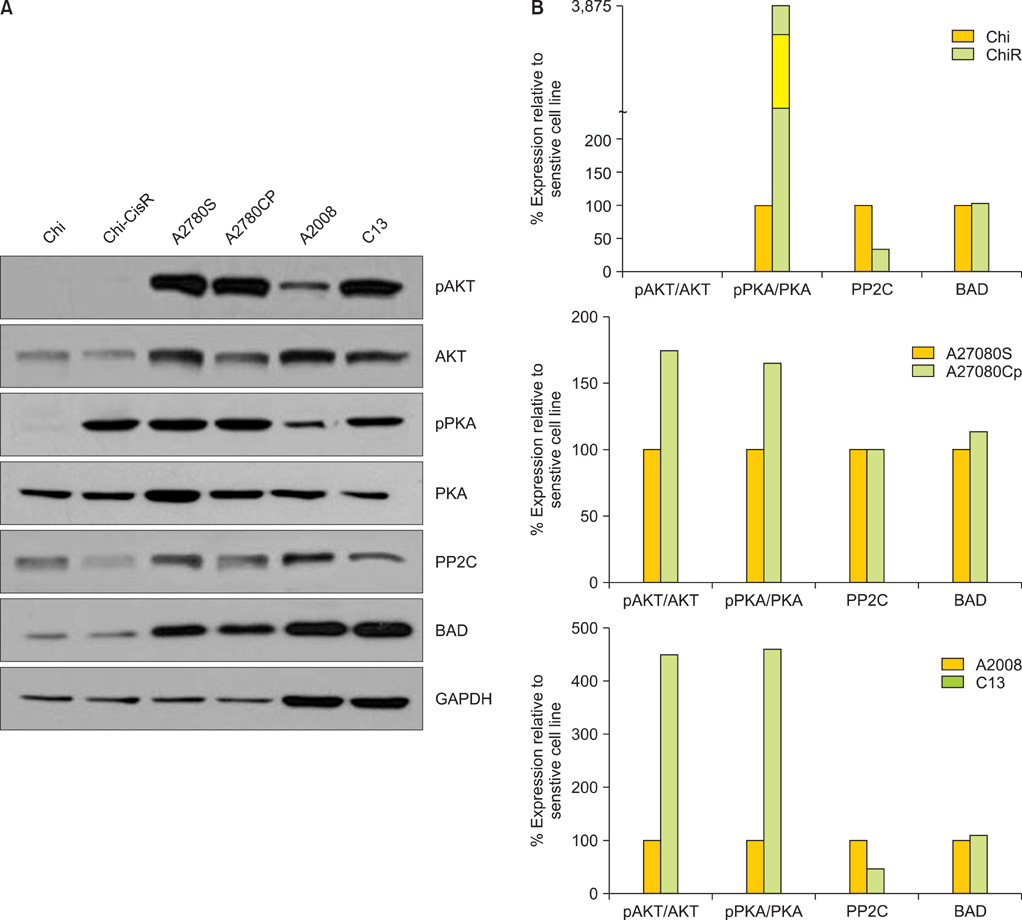
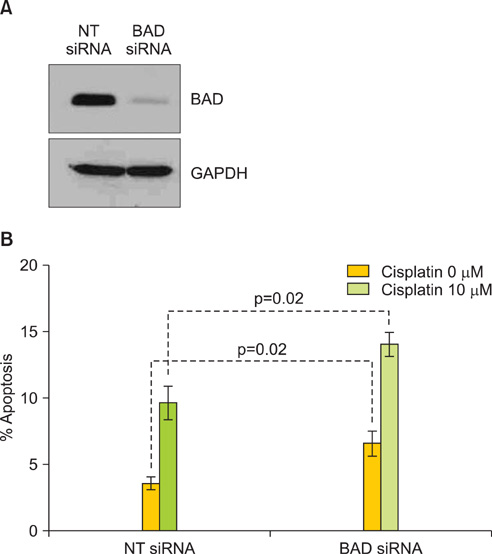
Protein levels of cyclin-dependent kinase 1 (CDK1) and protein phosphatase 2C (PP2C) were measured by immunofluorescence in a series of 64 primary advanced-stage serous OVCA patient samples. In parallel, levels of cAMP-dependent protein kinase (PKA), AKT, and PP2C were quantified by Western blot analysis in paired mother/daughter platinum-sensitive/resistant OVCA cell lines (A2008/C13, A2780S/A2780CP, Chi/ChiR). BAD pathway kinase CDK1 was depleted using siRNA transfection, and the influence on BAD phosphorylation and cisplatin-induced apoptosis was evaluated.
RESULTS
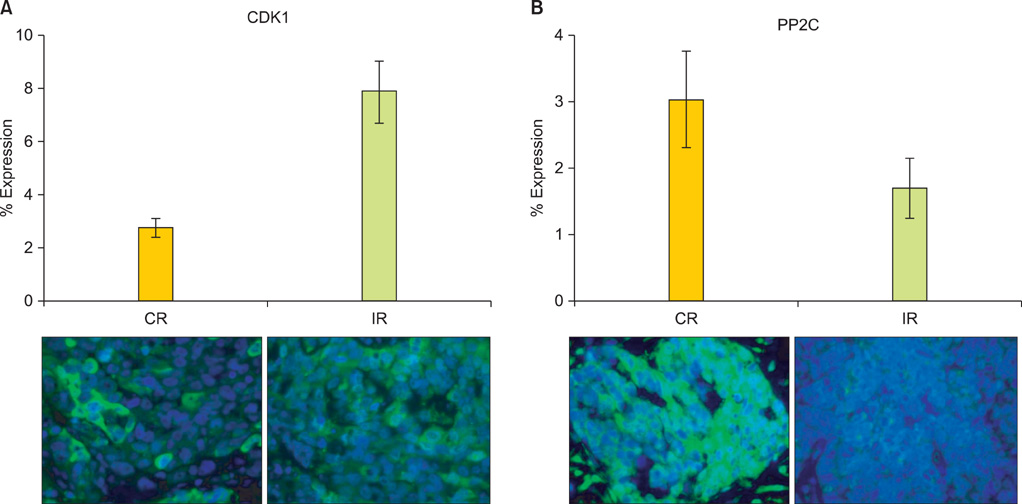
OVCA patient samples that demonstrated complete responses to primary platinum-based therapy demonstrated 4-fold higher CDK1 (p<0.0001) and 2-fold lower PP2C (p=0.14) protein levels than samples that demonstrated incomplete responses. Protein levels of PP2C were lower in the platinum-resistant versus that shown in the platinum-sensitive OVCA cell line sub-clones. Levels of PKA were higher in all platinum-resistant than in platinum-sensitive OVCA cell line sub-clones. Selective siRNA depletion of CDK1 increased sensitivity to cisplatin-induced apoptosis (p<0.002).
CONCLUSION
BAD pathway kinases and phosphatases, including CDK1 and PP2C, are associated with OVCA sensitivity to platinum and may represent therapeutic opportunities to enhance cytotoxic efficacy.
Keyword
MeSH Terms
-
Apoptosis
Blotting, Western
CDC2 Protein Kinase
Cell Death
Cell Line
Cisplatin
Cyclic AMP-Dependent Protein Kinases
Fluorescent Antibody Technique
Humans
Ovarian Neoplasms
Phosphoprotein Phosphatases
Phosphoric Monoester Hydrolases
Phosphorylation
Phosphotransferases
Platinum
Proteins
RNA, Small Interfering
Transfection
CDC2 Protein Kinase
Cisplatin
Cyclic AMP-Dependent Protein Kinases
Phosphoprotein Phosphatases
Phosphoric Monoester Hydrolases
Phosphotransferases
Platinum
Proteins
RNA, Small Interfering
Figure
Reference
-
1. Baker VV. Salvage therapy for recurrent epithelial ovarian cancer. Hematol Oncol Clin North Am. 2003. 17:977–988.2. Hansen HH, Eisenhauer EA, Hansen M, Neijt JP, Piccart MJ, Sessa C, et al. New cytostatic drugs in ovarian cancer. Ann Oncol. 1993. 4:Suppl 4. 63–70.3. Herrin VE, Thigpen JT. Chemotherapy for ovarian cancer: current concepts. Semin Surg Oncol. 1999. 17:181–188.4. Godwin AK, Meister A, O'Dwyer PJ, Huang CS, Hamilton TC, Anderson ME. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc Natl Acad Sci USA. 1992. 89:3070–3074.5. Johnson SW, Laub PB, Beesley JS, Ozols RF, Hamilton TC. Increased platinum-DNA damage tolerance is associated with cisplatin resistance and cross-resistance to various chemotherapeutic agents in unrelated human ovarian cancer cell lines. Cancer Res. 1997. 57:850–856.6. Johnson SW, Swiggard PA, Handel LM, Brennan JM, Godwin AK, Ozols RF, et al. Relationship between platinum-DNA adduct formation and removal and cisplatin cytotoxicity in cisplatin-sensitive and -resistant human ovarian cancer cells. Cancer Res. 1994. 54:5911–5916.7. Hayakawa J, Ohmichi M, Kurachi H, Kanda Y, Hisamoto K, Nishio Y, et al. Inhibition of BAD phosphorylation either at serine 112 via extracellular signal-regulated protein kinase cascade or at serine 136 via Akt cascade sensitizes human ovarian cancer cells to cisplatin. Cancer Res. 2000. 60:5988–5994.8. Mabuchi S, Ohmichi M, Kimura A, Hisamoto K, Hayakawa J, Nishio Y, et al. Inhibition of phosphorylation of BAD and Raf-1 by Akt sensitizes human ovarian cancer cells to paclitaxel. J Biol Chem. 2002. 277:33490–33500.9. Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981. 47:207–214.10. Rustin GJ, Nelstrop AE, Bentzen SM, Piccart MJ, Bertelsen K. Use of tumour markers in monitoring the course of ovarian cancer. Ann Oncol. 1999. 10:Suppl 1. 21–27.11. Rustin GJ, Nelstrop AE, McClean P, Brady MF, McGuire WP, Hoskins WJ, et al. Defining response of ovarian carcinoma to initial chemotherapy according to serum CA 125. J Clin Oncol. 1996. 14:1545–1551.12. Benedetti V, Perego P, Luca Beretta G, Corna E, Tinelli S, Righetti SC, et al. Modulation of survival pathways in ovarian carcinoma cell lines resistant to platinum compounds. Mol Cancer Ther. 2008. 7:679–687.13. Dressman HK, Berchuck A, Chan G, Zhai J, Bild A, Sayer R, et al. An integrated genomic-based approach to individualized treatment of patients with advanced-stage ovarian cancer. J Clin Oncol. 2007. 25:517–525.14. Jazaeri AA, Awtrey CS, Chandramouli GV, Chuang YE, Khan J, Sotiriou C, et al. Gene expression profiles associated with response to chemotherapy in epithelial ovarian cancers. Clin Cancer Res. 2005. 11:6300–6310.15. Potti A, Dressman HK, Bild A, Riedel RF, Chan G, Sayer R, et al. Genomic signatures to guide the use of chemotherapeutics. Nat Med. 2006. 12:1294–1300.16. Kim HK, Choi IJ, Kim CG, Kim HS, Oshima A, Michalowski A, et al. A gene expression signature of acquired chemoresistance to cisplatin and fluorouracil combination chemotherapy in gastric cancer patients. PLoS One. 2011. 6:e16694.17. Li M, Balch C, Montgomery JS, Jeong M, Chung JH, Yan P, et al. Integrated analysis of DNA methylation and gene expression reveals specific signaling pathways associated with platinum resistance in ovarian cancer. BMC Med Genomics. 2009. 2:34.18. Marchion DC, Cottrill HM, Xiong Y, Chen N, Bicaku E, Fulp WJ, et al. BAD phosphorylation determines ovarian cancer chemosensitivity and patient survival. Clin Cancer Res. 2011. 17:6356–6366.19. Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004. 116:205–219.20. Dejean LM, Martinez-Caballero S, Guo L, Hughes C, Teijido O, Ducret T, et al. Oligomeric Bax is a component of the putative cytochrome c release channel MAC, mitochondrial apoptosis-induced channel. Mol Biol Cell. 2005. 16:2424–2432.21. Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, et al. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J Cell Biol. 1999. 144:891–901.22. Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002. 111:331–342.23. Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995. 80:285–291.24. del Peso L, Gonzalez-Garcia M, Page C, Herrera R, Nunez G. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science. 1997. 278:687–689.25. Lizcano JM, Morrice N, Cohen P. Regulation of BAD by cAMP-dependent protein kinase is mediated via phosphorylation of a novel site, Ser155. Biochem J. 2000. 349(Pt 2):547–557.26. Tan Y, Demeter MR, Ruan H, Comb MJ. BAD Ser-155 phosphorylation regulates BAD/Bcl-XL interaction and cell survival. J Biol Chem. 2000. 275:25865–25869.27. Zhou XM, Liu Y, Payne G, Lutz RJ, Chittenden T. Growth factors inactivate the cell death promoter BAD by phosphorylation of its BH3 domain on Ser155. J Biol Chem. 2000. 275:25046–25051.28. Klumpp S, Selke D, Krieglstein J. Protein phosphatase type 2C dephosphorylates BAD. Neurochem Int. 2003. 42:555–560.29. Yang L, Omori K, Suzukawa J, Inagaki C. Calcineurin-mediated BAD Ser155 dephosphorylation in ammonia-induced apoptosis of cultured rat hippocampal neurons. Neurosci Lett. 2004. 357:73–75.30. Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997. 91:231–241.31. Datta SR, Ranger AM, Lin MZ, Sturgill JF, Ma YC, Cowan CW, et al. Survival factor-mediated BAD phosphorylation raises the mitochondrial threshold for apoptosis. Dev Cell. 2002. 3:631–643.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Apoptosis-related mRNA expression profiles of ovarian cancer cell lines following cisplatin treatment
- Expression of p53, p16, PTEN, and c-myc Gene with Cisplatin Treatment in Cisplatin Resistant Ovarian Cancer Cell Line
- SP1-induced lncRNA MCF2L-AS1 promotes cisplatin resistance in ovarian cancer by regulating IGF2BP1/IGF2/MEK/ERK axis
- The Effects of Mistletoe Extract and Anti-cancer Drugs on the Apoptosis of Gastric Cancer Cells
- A role of Cyclosporine A that suppresses multi-drug resistance (MDR) in the secondary chemotherapy drug resistant cell line of ovarian cancer