J Gynecol Oncol.
2010 Dec;21(4):255-261. 10.3802/jgo.2010.21.4.255.
Apoptosis-related mRNA expression profiles of ovarian cancer cell lines following cisplatin treatment
- Affiliations
-
- 1Department of Obstetrics and Gynecology, St. Vincent's Hospital, The Catholic University of Korea, Suwon, Korea.
- 2School of Inter-Disciplinary Bioscience and Bioengineering (I-Bio), Pohang University of Science and Technology (POSTECH), Pohang, Korea. kchoi@postech.ac.kr
- 3Division of Molecular and Life Sciences, Department of Life Science, Pohang University of Science and Technology (POSTECH), Pohang, Korea.
- KMID: 2173550
- DOI: http://doi.org/10.3802/jgo.2010.21.4.255
Abstract
OBJECTIVE
The aim of this study was to identify apoptosis-related genes of ovarian cancer cell lines following cisplatin treatment.
METHODS
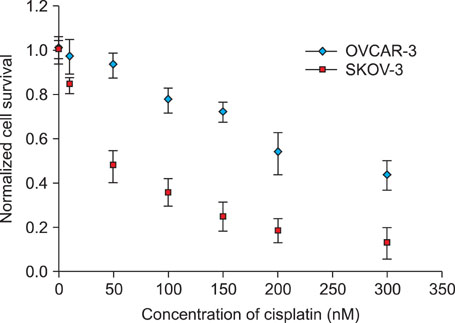
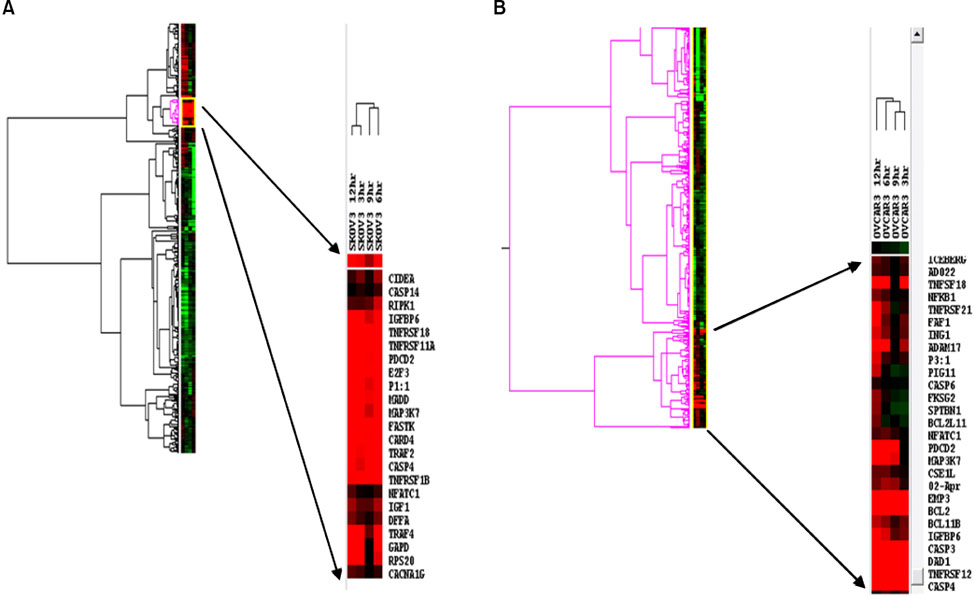
We used IC50 values and fluorescence-activated cell sorting analysis to compare cell death in 2 ovarian cancer cell lines, namely, SKOV-3 and OVCAR-3, upon treatment with cisplatin. Moreover, the change in transcriptional levels of apoptosis-associated genes was measured with a dendron-modified DNA microarray.
RESULTS
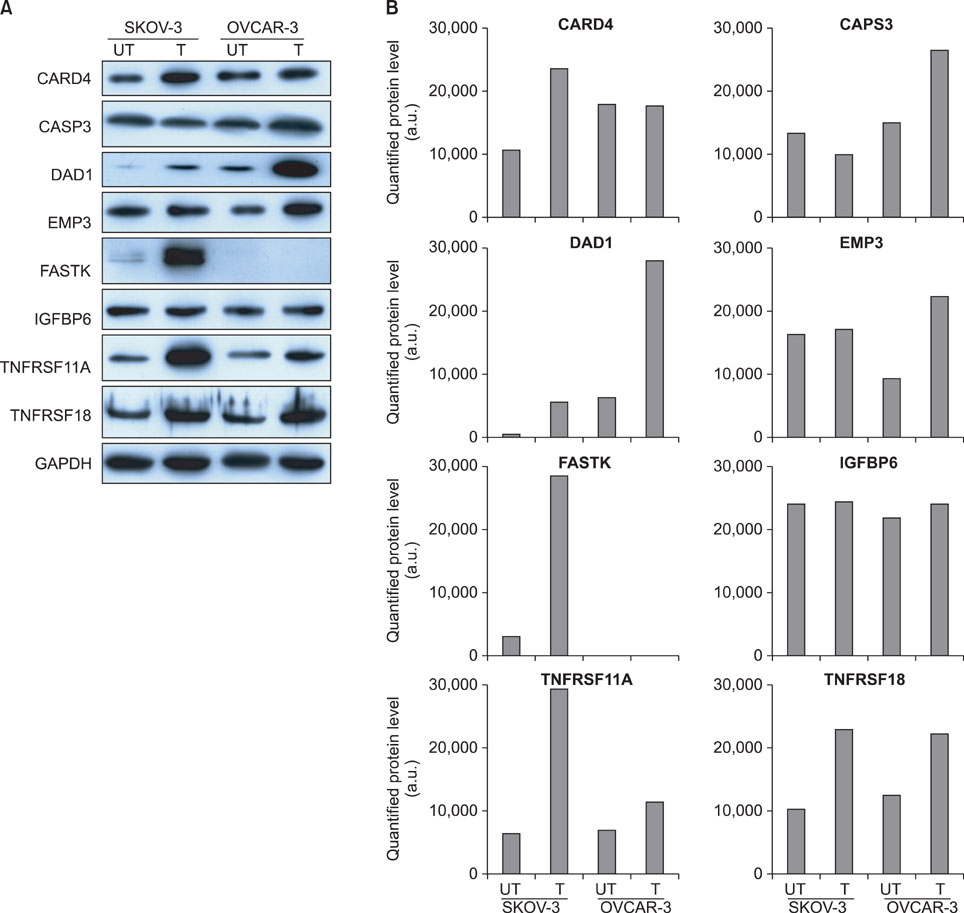
The protein levels for the up-regulated genes in each cell line were validated to identify the molecules that may determine the cellular behavior of cisplatin resistance. Eight genes were over-expressed in the 2 cell lines. The cisplatin-induced up-regulation of DAD1 in transcriptional and protein levels contributed to the cisplatin resistance of OVCAR-3, and the up-regulation of FASTK and TNFRSF11A in SKOV-3 resulted in its higher sensitivity to cisplatin than that of OVCAR-3.
CONCLUSION
In the present study, we have identified a set of genes responsible for apoptosis following cisplatin treatment in ovarian cancer cell lines. These genes may give information about the understanding of cisplatin-induced apoptosis in ovarian cancer.
MeSH Terms
Figure
Reference
-
1. Helm CW, States JC. Enhancing the efficacy of cisplatin in ovarian cancer treatment - could arsenic have a role. J Ovarian Res. 2009. 2:2.2. Giaccone G. Clinical perspectives on platinum resistance. Drugs. 2000. 59:Suppl 4. 9–17.3. Kartalou M, Essigmann JM. Mechanisms of resistance to cisplatin. Mutat Res. 2001. 478:23–43.4. Gately DP, Howell SB. Cellular accumulation of the anticancer agent cisplatin: a review. Br J Cancer. 1993. 67:1171–1176.5. Lanzi C, Perego P, Supino R, Romanelli S, Pensa T, Carenini N, et al. Decreased drug accumulation and increased tolerance to DNA damage in tumor cells with a low level of cisplatin resistance. Biochem Pharmacol. 1998. 55:1247–1254.6. Perego P, Caserini C, Gatti L, Carenini N, Romanelli S, Supino R, et al. A novel trinuclear platinum complex overcomes cisplatin resistance in an osteosarcoma cell system. Mol Pharmacol. 1999. 55:528–534.7. Mistry P, Kelland LR, Abel G, Sidhar S, Harrap KR. The relationships between glutathione, glutathione-S-transferase and cytotoxicity of platinum drugs and melphalan in eight human ovarian carcinoma cell lines. Br J Cancer. 1991. 64:215–220.8. Eastman A, Schulte N. Enhanced DNA repair as a mechanism of resistance to cis-diamminedichloroplatinum(II). Biochemistry. 1988. 27:4730–4734.9. Chu G. Cellular responses to cisplatin. The roles of DNA-binding proteins and DNA repair. J Biol Chem. 1994. 269:787–790.10. Anthoney DA, McIlwrath AJ, Gallagher WM, Edlin AR, Brown R. Microsatellite instability, apoptosis, and loss of p53 function in drug-resistant tumor cells. Cancer Res. 1996. 56:1374–1381.11. Aebi S, Kurdi-Haidar B, Gordon R, Cenni B, Zheng H, Fink D, et al. Loss of DNA mismatch repair in acquired resistance to cisplatin. Cancer Res. 1996. 56:3087–3090.12. Perego P, Righetti SC, Supino R, Delia D, Caserini C, Carenini N, et al. Role of apoptosis and apoptosis-related proteins in the cisplatin-resistant phenotype of human tumor cell lines. Apoptosis. 1997. 2:540–548.13. el-Deiry WS. Role of oncogenes in resistance and killing by cancer therapeutic agents. Curr Opin Oncol. 1997. 9:79–87.14. Kudoh K, Ramanna M, Ravatn R, Elkahloun AG, Bittner ML, Meltzer PS, et al. Monitoring the expression profiles of doxorubicin-induced and doxorubicin-resistant cancer cells by cDNA microarray. Cancer Res. 2000. 60:4161–4166.15. Johnsson A, Zeelenberg I, Min Y, Hilinski J, Berry C, Howell SB, et al. Identification of genes differentially expressed in association with acquired cisplatin resistance. Br J Cancer. 2000. 83:1047–1054.16. Johnsson A, Byrne P, de Bruin R, Weiner D, Wong J, Los G. Identification of gene clusters differentially expressed during the cellular injury responses (CIR) to cisplatin. Br J Cancer. 2001. 85:1206–1210.17. Black MM, Speer FD. Effects of cancer chemotherapeutic agents on dehydrogenase activity of human cancer tissue in vitro. Am J Clin Pathol. 1953. 23:218–227.18. Black MM, Speer FD. Further observations on the effects of cancer chemotherapeutic agents on the in vitro dehydrogenase activity of cancer tissue. J Natl Cancer Inst. 1954. 14:1147–1158.19. Goldie JH, Coldman AJ. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat Rep. 1979. 63:1727–1733.20. Iyer L, Ratain MJ. Pharmacogenetics and cancer chemotherapy. Eur J Cancer. 1998. 34:1493–1499.21. Cloven NG, Kyshtoobayeva A, Burger RA, Yu IR, Fruehauf JP. In vitro chemoresistance and biomarker profiles are unique for histologic subtypes of epithelial ovarian cancer. Gynecol Oncol. 2004. 92:160–166.22. Moch H, Schraml P, Bubendorf L, Mirlacher M, Kononen J, Gasser T, et al. High-throughput tissue microarray analysis to evaluate genes uncovered by cDNA microarray screening in renal cell carcinoma. Am J Pathol. 1999. 154:981–986.23. Wang Y, Rea T, Bian J, Gray S, Sun Y. Identification of the genes responsive to etoposide-induced apoptosis: application of DNA chip technology. FEBS Lett. 1999. 445:269–273.24. Elek J, Park KH, Narayanan R. Microarray-based expression profiling in prostate tumors. In Vivo. 2000. 14:173–182.25. Oh SJ, Cho SJ, Kim CO, Park JW. Characteristics of DNA microarrays fabricated on the various aminosilane layers. Langmuir. 2002. 18:1764–1769.26. Gao JH, He ZJ, Wang Q, Li X, Li YX, Liu M, et al. Low expression of S100P associated with paclitaxel resistance in ovarian cancer cell line. Chin Med J (Engl). 2008. 121:1563–1568.27. Oh SJ, Ju J, Kim BC, Ko E, Hong BJ, Park JG, et al. DNA microarrays on a dendron-modified surface improve significantly the detection of single nucleotide variations in the p53 gene. Nucleic Acids Res. 2005. 33:e90.28. Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, et al. Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res. 2002. 30:e15.29. Kelleher DJ, Gilmore R. DAD1, the defender against apoptotic cell death, is a subunit of the mammalian oligosaccharyltransferase. Proc Natl Acad Sci U S A. 1997. 94:4994–4999.30. Brewster JL, Martin SL, Toms J, Goss D, Wang K, Zachrone K, et al. Deletion of Dad1 in mice induces an apoptosis-associated embryonic death. Genesis. 2000. 26:271–278.31. Tian Q, Taupin J, Elledge S, Robertson M, Anderson P. Fas-activated serine/threonine kinase (FAST) phosphorylates TIA-1 during Fas-mediated apoptosis. J Exp Med. 1995. 182:865–874.32. Hughes AE, Ralston SH, Marken J, Bell C, MacPherson H, Wallace RG, et al. Mutations in TNFRSF11A, affecting the signal peptide of RANK, cause familial expansile osteolysis. Nat Genet. 2000. 24:45–48.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of p53, p16, PTEN, and c-myc Gene with Cisplatin Treatment in Cisplatin Resistant Ovarian Cancer Cell Line
- Study on Measure of Chemosensitivities to Topotecan, Cisplatin and Taxol Theraphy in Ovarian Cancer Cell Lines: Relationship with p53 and bcl-2 Expression and Apoptosis
- Tasquinimod promotes the sensitivity of ovarian cancer cells to cisplatin by down-regulating the HDAC4/p21 pathway
- Expression of Genes Related to Multidrug Resistance and Apoptosis in Human Ovarian Cancer Cell Lines, Sensitive and Resistant to Cisplatin
- Epigenetic modification of α-N-acetylgalactosaminidase enhances cisplatin resistance in ovarian cancer