J Breast Cancer.
2009 Dec;12(4):241-248. 10.4048/jbc.2009.12.4.241.
Comparison between Therapeutic Efficacies of Histone Deacetylase Inhibitors and Established Drug Regimens Against Breast Cancer Cells using the Histoculture Drug Response Assay
- Affiliations
-
- 1Department of Surgery, University of Ulsan College of Medicin, Seoul, Korea. jckim@amc.seoul.kr
- 2Institute of Innovative Cancer Research, Asan Institute for Life Sciences and Asan Medical Center, Seoul, Korea.
- 3Division of Medical Genetics, Korea Research Institute of Bioscience & Biotechnology, Daejeon, Korea.
- 4Department of Internal Medicine, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2175513
- DOI: http://doi.org/10.4048/jbc.2009.12.4.241
Abstract
- PURPOSE
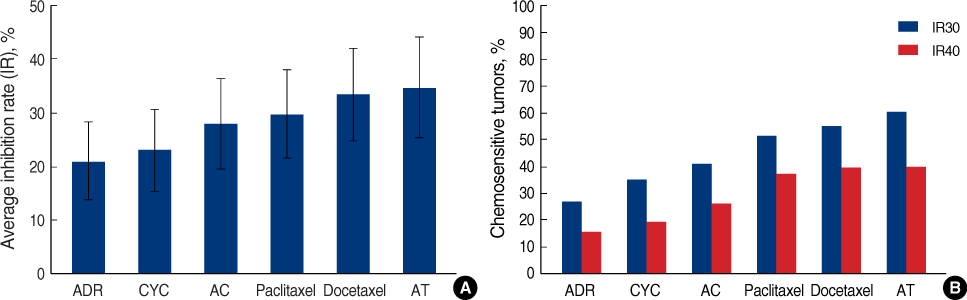
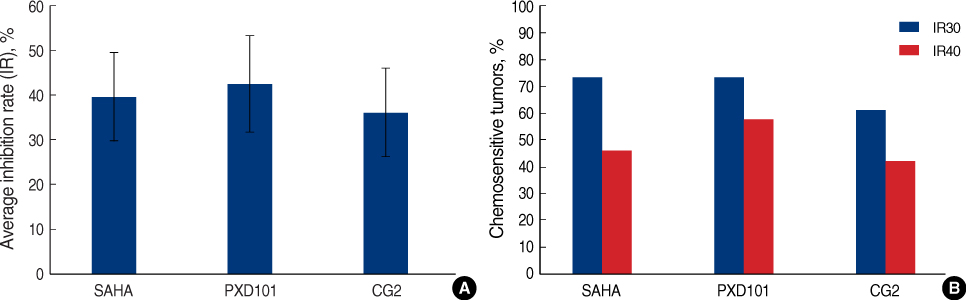
Histone deacetylase inhibitors (HDACIs) induce accumulation of acetylated histones in nucleosomes, which lead to reactivate gene expression and inhibit the growth and survival of tumor cells. This study evaluated the efficacy of HDACIs in breast cancer cells in comparison with other established drug regimens. METHODS: Drug responses of tumor samples from mastectomy specimens of 78 breast cancer patients were evaluated using the histoculture drug response assay (HDRA). Tumor inhibition rates (IRs) of established drug regimens such as doxorubicin, cyclophosphamide, doxorubicin with cyclophosphamide (AC), paclitaxel, docetaxel and doxorubicin with docetaxel (AT), as well as those of three HDACIs (SAHA, PXD101, and a novel compound CG-2) were evaluate. RESULTS: The percentages of chemosensitive tumors (chemoresponsiveness) were 26.9-60.3% with established regimens and 61.5-73.1% with HDACIs when the cutoff value for inhibition rate was set at 30%. Breast cancer cells appeared to be more chemoresponsive to HDACIs than to established drug regimens. Chemoresponsiveness to AT was the highest among the established drug regimens. A combination regimen offered higher activity than did a single drug (doxorubicin vs AT; p<0.001). HER2/Neu-overexpressing breast cancers were chemosensitive to SAHA and AT (p=0.031 and 0.04, respectively). CONCLUSION: Our findings show that breast cancer cells were sensitive to HDACIs, with therapeutic efficacies comparable to those of established drug regimens. Specific biological markers such as HER2/Neu could be assessed for effectiveness as HDACIs chemosensitivity markers in further clinical trials.
Keyword
MeSH Terms
-
Biomarkers
Breast
Breast Neoplasms
Cyclophosphamide
Doxorubicin
Gene Expression
Histone Deacetylase Inhibitors
Histone Deacetylases
Histones
Humans
Hydroxamic Acids
Mastectomy
Nucleosomes
Paclitaxel
Sulfonamides
Taxoids
Cyclophosphamide
Doxorubicin
Histone Deacetylase Inhibitors
Histone Deacetylases
Histones
Hydroxamic Acids
Nucleosomes
Paclitaxel
Sulfonamides
Taxoids
Figure
Reference
-
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005. 55:74–108.
Article2. Yamashiro H, Toi M. Update of evidence in chemotherapy for breast cancer. Int J Clin Oncol. 2008. 13:3–7.
Article3. Laird PW. Cancer epigenetics. Hum Mol Genet. 2005. 14 Spec No 1:R65–R76.
Article4. Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001. 1:194–202.
Article5. Marchion D, Münster P. Development of histone deacetylase inhibitors for cancer treatment. Expert Rev Anticancer Ther. 2007. 7:583–598.
Article6. Glaser KB. HDAC inhibitors: clinical update and mechanism-based potential. Biochem Pharmacol. 2007. 74:659–671.
Article7. Munster PN, Troso-Sandoval T, Rosen N, Rifkind R, Marks PA, Richon VM. The histone deacetylase inhibitor suberoylanilide hydroxamic acid induces differentiation of human breast cancer cells. Cancer Res. 2001. 61:8492–8497.8. Luu TH, Morgan RJ, Leong L, Lim D, McNamara M, Portnow J, et al. A phase II trial of vorinostat (suberoylanilide hydroxamic acid) in metastatic breast cancer: a California Cancer Consortium study. Clin Cancer Res. 2008. 14:7138–7142.
Article9. Hirano Y, Ushiyama T, Suzuki K, Fujita K. Clinical application of an in vitro chemosensitivity test, the Histoculture Drug Response Assay, to urological cancers: wide distribution of inhibition rates in bladder cancer and renal cell cancer. Urol Res. 1999. 27:483–488.
Article10. Ohie S, Udagawa Y, Aoki D, Nozawa S. Histoculture drug response assay to monitor chemoresponse. Methods Mol Med. 2005. 110:79–86.
Article11. Furukawa T, Kubota T, Hoffman RM. Clinical applications of the histoculture drug response assay. Clin Cancer Res. 1995. 1:305–311.12. Kubota T, Sasano N, Abe O, Nakao I, Kawamura E, Saito T, et al. Potential of the histoculture drug-response assay to contribute to cancer patient survival. Clin Cancer Res. 1995. 1:1537–1543.13. Ariyoshi Y, Shimahara M, Tanigawa N. Study on chemosensitivity of oral squamous cell carcinomas by histoculture drug response assay. Oral Oncol. 2003. 39:701–707.
Article14. Yamauchi M, Satta T, Ito A, Kondo T, Takagi H. A feasibility study of the SDI test for the evaluation of gastrointestinal cancer sensitivity to anticancer drugs. J Surg Oncol. 1991. 47:253–260.
Article15. Yu LJ, Drewes P, Gustafsson K, Brain EG, Hecht JE, Waxman DJ. In vivo modulation of alternative pathways of P-450-catalyzed cyclophosphamide metabolism: impact on pharmacokinetics and antitumor activity. J Pharmacol Exp Ther. 1999. 288:928–937.16. Palmeri S, Leonardi V, Tamburo De Bella M, Morabito A, Vaglica M, Accurso V, et al. Doxorubicin-docetaxel sequential schedule: results of front-line treatment in advanced breast cancer. Oncology. 2002. 63:205–212.
Article17. Perez EA. Adjuvant therapy approaches to breast cancer: should taxanes be incorporated? Curr Oncol Rep. 2003. 5:66–71.
Article18. Carnesecchi S, Bras-Goncalves R, Bradaia A, Zeisel M, Gossé F, Poupon MF, et al. Geraniol, a component of plant essential oils, modulates DNA synthesis and potentiates 5-fluorouracil efficacy on human colon tumor xenografts. Cancer Lett. 2004. 215:53–59.
Article19. Kim JC, Kim DD, Lee YM, Kim TW, Cho DH, Kim MB, et al. Evaluation of novel histone deacetylase inhibitors as therapeutic agents for colorectal adenocarcinomas compared to established regimens with the histoculture drug response assay. Int J Colorectal Dis. 2009. 24:209–218.
Article20. Hayon T, Dvilansky A, Shpilberg O, Nathan I. Appraisal of the MTT-based assay as a useful tool for predicting drug chemosensitivity in leukemia. Leuk Lymphoma. 2003. 44:1957–1962.
Article21. Singh B, Li R, Xu L, Poluri A, Patel S, Shaha AR, et al. Prediction of survival in patients with head and neck cancer using the histoculture drug response assay. Head Neck. 2002. 24:437–442.
Article22. Jung YS, Cho YU, Suh YJ, Kim JS, Oh SJ, Lim CW, et al. Can the histoculture drug response assay predict the clinical results of chemotherapy in breast cancer? J Breast Cancer. 2007. 10:193–198.
Article23. Burgess A, Ruefli A, Beamish H, Warrener R, Saunders N, Johnstone R, et al. Histone deacetylase inhibitors specifically kill nonproliferating tumour cells. Oncogene. 2004. 23:6693–6701.
Article24. Qiu L, Burgess A, Fairlie DP, Leonard H, Parsons PG, Gabrielli BG. Histone deacetylase inhibitors trigger a G2 checkpoint in normal cells that is defective in tumor cells. Mol Biol Cell. 2000. 11:2069–2083.
Article25. Kim MS, Blake M, Baek JH, Kohlhagen G, Pommier Y, Carrier F. Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Res. 2003. 63:7291–7300.26. Tumber A, Collins LS, Petersen KD, Thougaard A, Christiansen SJ, Dejligbjerg M, et al. The histone deacetylase inhibitor PXD101 synergises with 5-fluorouracil to inhibit colon cancer cell growth in vitro and in vivo. Cancer Chemother Pharmacol. 2007. 60:275–283.
Article27. Marchion DC, Bicaku E, Daud AI, Richon V, Sullivan DM, Munster PN. Sequence-specific potentiation of topoisomerase II inhibitors by the histone deacetylase inhibitor suberoylanilide hydroxamic acid. J Cell Biochem. 2004. 92:223–237.
Article28. Fuino L, Bali P, Wittmann S, Donapaty S, Guo F, Yamaguchi H, et al. Histone deacetylase inhibitor LAQ824 down-regulates Her-2 and sensitizes human breast cancer cells to trastuzumab, taxotere, gemcitabine, and epothilone B. Mol Cancer Ther. 2003. 2:971–984.29. Bali P, Pranpat M, Swaby R, Fiskus W, Yamaguchi H, Balasis M, et al. Activity of suberoylanilide hydroxamic Acid against human breast cancer cells with amplification of her-2. Clin Cancer Res. 2005. 11:6382–6389.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- HDAC and HDAC Inhibitor: From Cancer to Cardiovascular Diseases
- Sequence-Dependent Radiosensitization of Histone Deacetylase Inhibitors Trichostatin A and SK-7041
- Anti-Cancer Effect of 3-(4-dimethylamino phenyl)-N-hydroxy-2-propenamide in MCF-7 Human Breast Cancer
- Epigenetic Modifications: Novel Therapeutic Approach for Thyroid Cancer
- The Reliability of Histoculture Drug Response Assay (HDRA) in Chemosensitivity Tests for Breast Cancer