Cancer Res Treat.
2013 Dec;45(4):334-342.
Sequence-Dependent Radiosensitization of Histone Deacetylase Inhibitors Trichostatin A and SK-7041
- Affiliations
-
- 1Department of Radiation Oncology, Seoul National University College of Medicine, Seoul, Korea. ihkim@snu.ac.kr
- 2Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 3Institute of Radiation Medicine, Medical Research Center, Seoul National University, Seoul, Korea.
- 4Department of Radiation Oncology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
Abstract
- PURPOSE
This preclinical study is to determine whether the capacity of histone deacetylase (HDAC) inhibitors to enhance radiation response depends on temporal sequences of HDAC inhibition and irradiation.
MATERIALS AND METHODS
The effects of HDAC inhibitors trichostatin A (TSA) and SK-7041 on radiosensitivity in human lung cancer cells were examined using a clonogenic assay, exposing cells to HDAC inhibitors in various sequences of HDAC inhibition and radiation. We performed Western blot of acetylated histone H3 and flow cytometry to analyze cell cycle phase distribution.
RESULTS
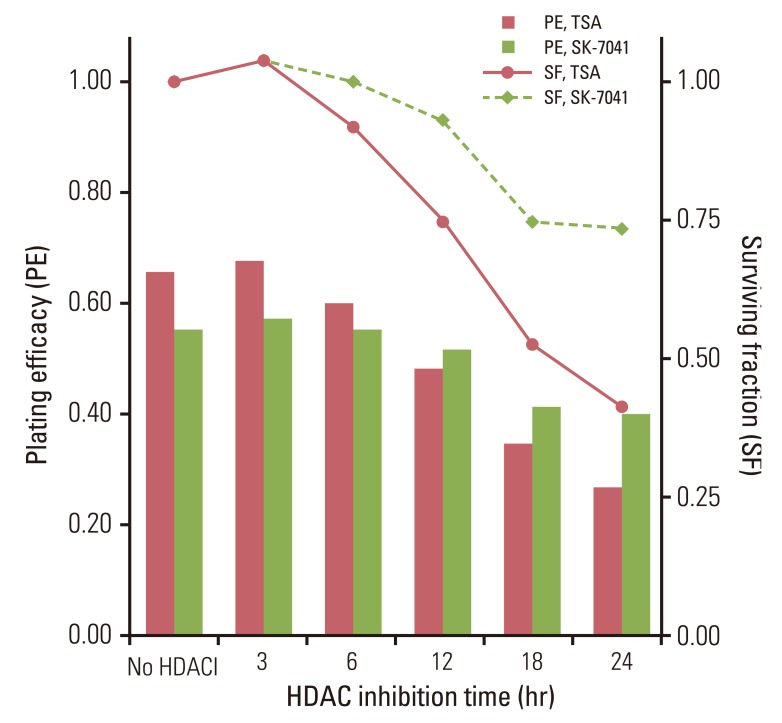
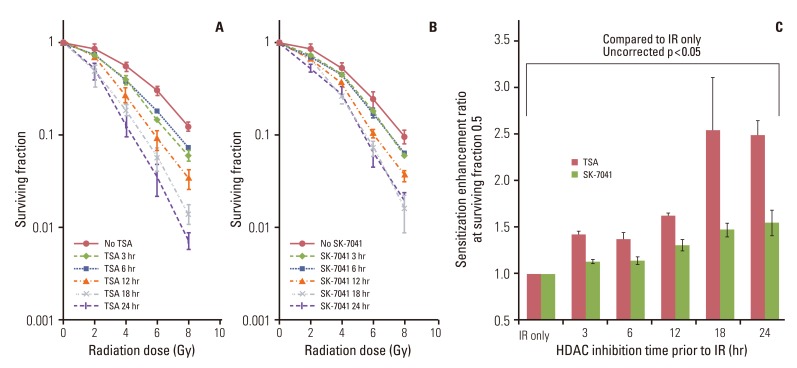
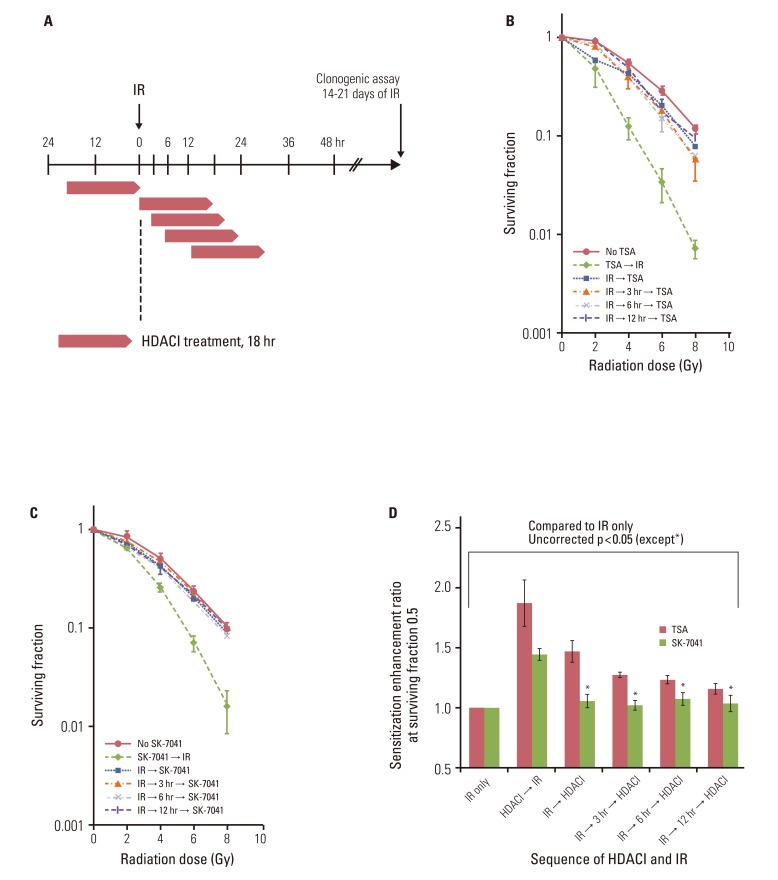
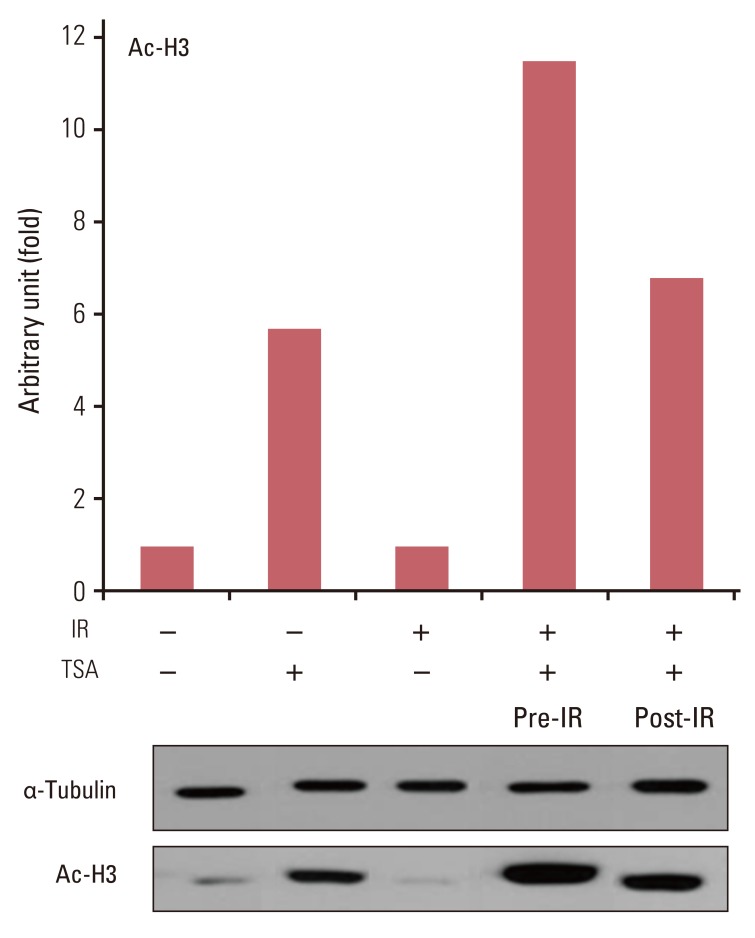
TSA and SK-7041 augmented radiation cell lethality in an exposure time-dependent manner when delivered before irradiation. The impact of TSA and SK-7041 on radiosensitivity rapidly diminished when HDAC inhibition was delayed after irradiation. Radiation induced the acetylation of histone H3 in cells exposed to TSA, while irradiation alone had no effect on the expression of acetylated histone H3 in TSA-naive cells. Preirradiation exposure to TSA abrogated radiation-induced G2/M-phase arrest. When delivered after irradiation, TSA had no effect on the peak of radiation-induced G2/M-phase arrest.
CONCLUSION
TSA and SK-7041 enhances radiosensitivity only when delivered before irradiation. Unless proven otherwise, it seems prudent to apply scheduling including preirradiation HDAC inhibition so that maximal radiosensitization is obtained.
MeSH Terms
Figure
Reference
-
1. Hassig CA, Schreiber SL. Nuclear histone acetylases and deacetylases and transcriptional regulation: HATs off to HDACs. Curr Opin Chem Biol. 1997; 1:300–308. PMID: 9667866.
Article2. Khan O, La Thangue NB. HDAC inhibitors in cancer biology: emerging mechanisms and clinical applications. Immunol Cell Biol. 2012; 90:85–94. PMID: 22124371.
Article3. Shabason JE, Tofilon PJ, Camphausen K. Grand rounds at the National Institutes of Health: HDAC inhibitors as radiation modifiers, from bench to clinic. J Cell Mol Med. 2011; 15:2735–2744. PMID: 21362133.
Article4. Camphausen K, Tofilon PJ. Inhibition of histone deacetylation: a strategy for tumor radiosensitization. J Clin Oncol. 2007; 25:4051–4056. PMID: 17827453.
Article5. Cerna D, Camphausen K, Tofilon PJ. Histone deacetylation as a target for radiosensitization. Curr Top Dev Biol. 2006; 73:173–204. PMID: 16782459.
Article6. Adimoolam S, Sirisawad M, Chen J, Thiemann P, Ford JM, Buggy JJ. HDAC inhibitor PCI-24781 decreases RAD51 expression and inhibits homologous recombination. Proc Natl Acad Sci U S A. 2007; 104:19482–19487. PMID: 18042714.
Article7. Chinnaiyan P, Vallabhaneni G, Armstrong E, Huang SM, Harari PM. Modulation of radiation response by histone deacetylase inhibition. Int J Radiat Oncol Biol Phys. 2005; 62:223–229. PMID: 15850925.
Article8. Kim IA, Shin JH, Kim IH, Kim JH, Kim JS, Wu HG, et al. Histone deacetylase inhibitor-mediated radiosensitization of human cancer cells: class differences and the potential influence of p53. Clin Cancer Res. 2006; 12(3 Pt 1):940–949. PMID: 16467109.
Article9. Kim JH, Shin JH, Kim IH. Susceptibility and radiosensitization of human glioblastoma cells to trichostatin A, a histone deacetylase inhibitor. Int J Radiat Oncol Biol Phys. 2004; 59:1174–1180. PMID: 15234053.
Article10. Knipstein JA, Birks DK, Donson AM, Alimova I, Foreman NK, Vibhakar R. Histone deacetylase inhibition decreases proliferation and potentiates the effect of ionizing radiation in atypical teratoid/rhabdoid tumor cells. Neuro Oncol. 2012; 14:175–183. PMID: 22156471.
Article11. Camphausen K, Burgan W, Cerra M, Oswald KA, Trepel JB, Lee MJ, et al. Enhanced radiation-induced cell killing and prolongation of gammaH2AX foci expression by the histone deacetylase inhibitor MS-275. Cancer Res. 2004; 64:316–321. PMID: 14729640.12. Camphausen K, Cerna D, Scott T, Sproull M, Burgan WE, Cerra MA, et al. Enhancement of in vitro and in vivo tumor cell radiosensitivity by valproic acid. Int J Cancer. 2005; 114:380–386. PMID: 15578701.13. Chinnaiyan P, Cerna D, Burgan WE, Beam K, Williams ES, Camphausen K, et al. Postradiation sensitization of the histone deacetylase inhibitor valproic acid. Clin Cancer Res. 2008; 14:5410–5415. PMID: 18765532.
Article14. Park JH, Jung Y, Kim TY, Kim SG, Jong HS, Lee JW, et al. Class I histone deacetylase-selective novel synthetic inhibitors potently inhibit human tumor proliferation. Clin Cancer Res. 2004; 10:5271–5281. PMID: 15297431.
Article15. Kim IA, Kim IH, Kim HJ, Chie EK, Kim JS. HDAC inhibitor-mediated radiosensitization in human carcinoma cells: a general phenomenon? J Radiat Res. 2010; 51:257–263. PMID: 20505264.
Article16. Zhang Y, Adachi M, Zou H, Hareyama M, Imai K, Shinomura Y. Histone deacetylase inhibitors enhance phosphorylation of histone H2AX after ionizing radiation. Int J Radiat Oncol Biol Phys. 2006; 65:859–866. PMID: 16751067.
Article17. Van Nifterik KA, Van den Berg J, Slotman BJ, Lafleur MV, Sminia P, Stalpers LJ. Valproic acid sensitizes human glioma cells for temozolomide and gamma-radiation. J Neurooncol. 2012; 107:61–67. PMID: 22037799.18. Kim GD, Choi YH, Dimtchev A, Jeong SJ, Dritschilo A, Jung M. Sensing of ionizing radiation-induced DNA damage by ATM through interaction with histone deacetylase. J Biol Chem. 1999; 274:31127–31130. PMID: 10531300.
Article19. Kao GD, McKenna WG, Guenther MG, Muschel RJ, Lazar MA, Yen TJ. Histone deacetylase 4 interacts with 53BP1 to mediate the DNA damage response. J Cell Biol. 2003; 160:1017–1027. PMID: 12668657.
Article20. Sun Y, Jiang X, Chen S, Fernandes N, Price BD. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc Natl Acad Sci U S A. 2005; 102:13182–13187. PMID: 16141325.
Article21. Sun Y, Jiang X, Chen S, Price BD. Inhibition of histone acetyltransferase activity by anacardic acid sensitizes tumor cells to ionizing radiation. FEBS Lett. 2006; 580:4353–4356. PMID: 16844118.
Article22. Bernhard EJ, Maity A, Muschel RJ, McKenna WG. Effects of ionizing radiation on cell cycle progression. A review. Radiat Environ Biophys. 1995; 34:79–83. PMID: 7652155.23. Playle LC, Hicks DJ, Qualtrough D, Paraskeva C. Abrogation of the radiation-induced G2 checkpoint by the staurosporine derivative UCN-01 is associated with radiosensitisation in a subset of colorectal tumour cell lines. Br J Cancer. 2002; 87:352–358. PMID: 12177808.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In vivo Radiosensitization Effect of HDAC Inhibitor, SK-7041 on RIF-1 Cell Line
- Activation of ATM-dependent DNA Damage Signal Pathway by a Histone Deacetylase Inhibitor, Trichostatin A
- Role of histone deacetylase activity in the developing lateral line neuromast of zebrafish larvae
- A Histone Deacetylase Inhibitor, Trichostatin A, Enhances Radiosensitivity by Abrogating G2/M Arrest in Human Carcinoma Cells
- Trichostatin A, a Histone Deacetylase Inhibitor, Potentiated Cytotoxic Effect of Ionizing Radiation in Human Head and Neck Cancer Cell Lines