Endocrinol Metab.
2017 Sep;32(3):326-331. 10.3803/EnM.2017.32.3.326.
Epigenetic Modifications: Novel Therapeutic Approach for Thyroid Cancer
- Affiliations
-
- 1Laboratory of Molecular Biology, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA. chengs@mail.nih.gov
- KMID: 2389812
- DOI: http://doi.org/10.3803/EnM.2017.32.3.326
Abstract
- The incidence of thyroid cancer is growing the fastest among all cancers in the United States, especially in women. The number of patients with thyroid neoplasm is part of an even larger number of patients who often need to undergo an operation to exclude a cancer diagnosis. While differentiated thyroid cancer (papillary thyroid cancer and follicular thyroid cancer) accounts for most cases of thyroid cancer and has a relatively good prognosis, effective treatments for patients with de-differentiated and anaplastic thyroid cancer are still gravely needed. Despite progress in the identification of genetic changes in thyroid cancer, the impact of aberrant epigenetic alterations on thyroid cancer remains to be fully elucidated. Understanding of the roles of epigenetic changes in thyroid cancer could open new opportunities for the identification of innovative molecular targets for novel treatment modalities, especially for anaplastic thyroid cancer for which treatment is very limited. This article briefly reviews the studies that exemplify the potential for and promise of using epigenetic regulators in the treatment of thyroid cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes Dev. 2011; 25:1010–1022.2. Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet. 2002; 3:415–428.3. Peterson CL, Laniel MA. Histones and histone modifications. Curr Biol. 2004; 14:R546–R551.4. Asa SL, Ezzat S. The epigenetic landscape of differentiated thyroid cancer. Mol Cell Endocrinol. 2017; 07. 12. [Epub]. DOI: 10.1016/j.mce.2017.07.012.5. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest. 2016; 126:1052–1066.6. Kim WG, Zhu X, Kim DW, Zhang L, Kebebew E, Cheng SY. Reactivation of the silenced thyroid hormone receptor β gene expression delays thyroid tumor progression. Endocrinology. 2013; 154:25–35.7. Moraes L, Galrao AL, Rubio I, Cerutti JM. Transcriptional regulation of the potential tumor suppressor ABI3 gene in thyroid carcinomas: interplay between methylation and NKX2-1 availability. Oncotarget. 2016; 7:25960–25970.8. Latini FR, Hemerly JP, Freitas BC, Oler G, Riggins GJ, Cerutti JM. ABI3 ectopic expression reduces in vitro and in vivo cell growth properties while inducing senescence. BMC Cancer. 2011; 11:11.9. Chi P, Allis CD, Wang GG. Covalent histone modifications: miswritten, misinterpreted and mis-erased in human cancers. Nat Rev Cancer. 2010; 10:457–469.10. Noureen N, Rashid H, Kalsoom S. Identification of type-specific anticancer histone deacetylase inhibitors: road to success. Cancer Chemother Pharmacol. 2010; 66:625–633.11. Woyach JA, Kloos RT, Ringel MD, Arbogast D, Collamore M, Zwiebel JA, et al. Lack of therapeutic effect of the histone deacetylase inhibitor vorinostat in patients with metastatic radioiodine-refractory thyroid carcinoma. J Clin Endocrinol Metab. 2009; 94:164–170.12. Sherman EJ, Su YB, Lyall A, Schoder H, Fury MG, Ghossein RA, et al. Evaluation of romidepsin for clinical activity and radioactive iodine reuptake in radioactive iodine-refractory thyroid carcinoma. Thyroid. 2013; 23:593–599.13. Zhu X, Kim DW, Zhao L, Willingham MC, Cheng SY. SAHA-induced loss of tumor suppressor Pten gene promotes thyroid carcinogenesis in a mouse model. Endocr Relat Cancer. 2016; 23:521–533.14. Fedier A, Dedes KJ, Imesch P, Von Bueren AO, Fink D. The histone deacetylase inhibitors suberoylanilide hydroxamic (Vorinostat) and valproic acid induce irreversible and MDR1-independent resistance in human colon cancer cells. Int J Oncol. 2007; 31:633–641.15. Fantin VR, Loboda A, Paweletz CP, Hendrickson RC, Pierce JW, Roth JA, et al. Constitutive activation of signal transducers and activators of transcription predicts vorinostat resistance in cutaneous T-cell lymphoma. Cancer Res. 2008; 68:3785–3794.16. Shao W, Growney JD, Feng Y, O'Connor G, Pu M, Zhu W, et al. Activity of deacetylase inhibitor panobinostat (LBH589) in cutaneous T-cell lymphoma models: defining molecular mechanisms of resistance. Int J Cancer. 2010; 127:2199–2208.17. Lee JH, Choy ML, Marks PA. Mechanisms of resistance to histone deacetylase inhibitors. Adv Cancer Res. 2012; 116:39–86.18. Eckschlager T, Plch J, Stiborova M, Hrabeta J. Histone deacetylase inhibitors as anticancer drugs. Int J Mol Sci. 2017; 18:E1414.19. Radwan M, Serya R. Fragment-based drug discovery in the bromodomain and extra-terminal domain family. Arch Pharm (Weinheim). 2017; 350:e1700147.20. Asangani IA, Dommeti VL, Wang X, Malik R, Cieslik M, Yang R, et al. Therapeutic targeting of BET bromodomain proteins in castration-resistant prostate cancer. Nature. 2014; 510:278–282.21. Cheng Z, Gong Y, Ma Y, Lu K, Lu X, Pierce LA, et al. Inhibition of BET bromodomain targets genetically diverse glioblastoma. Clin Cancer Res. 2013; 19:1748–1759.22. Lockwood WW, Zejnullahu K, Bradner JE, Varmus H. Sensitivity of human lung adenocarcinoma cell lines to targeted inhibition of BET epigenetic signaling proteins. Proc Natl Acad Sci U S A. 2012; 109:19408–19413.23. Shimamura T, Chen Z, Soucheray M, Carretero J, Kikuchi E, Tchaicha JH, et al. Efficacy of BET bromodomain inhibition in Kras-mutant non-small cell lung cancer. Clin Cancer Res. 2013; 19:6183–6192.24. Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, et al. Selective inhibition of BET bromodomains. Nature. 2010; 468:1067–1073.25. Loven J, Hoke HA, Lin CY, Lau A, Orlando DA, Vakoc CR, et al. Selective inhibition of tumor oncogenes by disruption of super-enhancers. Cell. 2013; 153:320–334.26. Enomoto K, Zhu X, Park S, Zhao L, Zhu YJ, Willingham MC, et al. Targeting MYC as a therapeutic intervention for anaplastic thyroid cancer. J Clin Endocrinol Metab. 2017; 102:2268–2280.27. Zhu X, Zhao L, Park JW, Willingham MC, Cheng SY. Synergistic signaling of KRAS and thyroid hormone receptor β mutants promotes undifferentiated thyroid cancer through MYC up-regulation. Neoplasia. 2014; 16:757–769.28. Zhu X, Enomoto K, Zhao L, Zhu YJ, Willingham MC, Meltzer P, et al. Bromodomain and extraterminal protein inhibitor JQ1 suppresses thyroid tumor growth in a mouse model. Clin Cancer Res. 2017; 23:430–440.29. Marlow LA, D'Innocenzi J, Zhang Y, Rohl SD, Cooper SJ, Sebo T, et al. Detailed molecular fingerprinting of four new anaplastic thyroid carcinoma cell lines and their use for verification of RhoB as a molecular therapeutic target. J Clin Endocrinol Metab. 2010; 95:5338–5347.30. Reeb AN, Li W, Lin RY. Bioluminescent human thyrospheres allow noninvasive detection of anaplastic thyroid cancer growth and metastases in vivo. Thyroid. 2014; 24:1134–1138.31. Marlow LA, Bok I, Smallridge RC, Copland JA. RhoB upregulation leads to either apoptosis or cytostasis through differential target selection. Endocr Relat Cancer. 2015; 22:777–792.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Genetic and epigenetic alterations of colorectal cancer
- Epigenetic Cross-Talk between DNA Methylation and Histone Modifications in Human Cancers
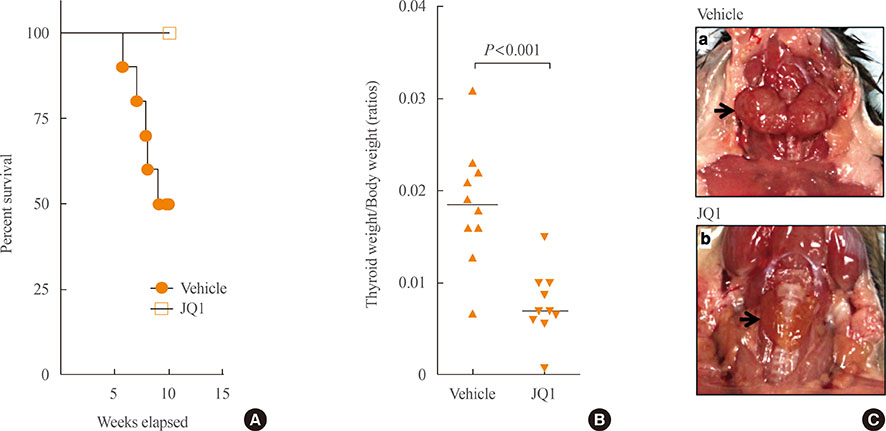
- Pathogenesis and biomarkers of colorectal cancer by epigenetic alteration
- Recent Clinical Update of Acute Myeloid Leukemia: Focus on Epigenetic Therapies
- Researches of Epigenetic Epidemiology for Infections and Radiation as Carcinogen